A kind of aspergillus oryzae phospholipase c and its coding gene and application
A phospholipase and phosphatidylcholine technology, which can be used in application, genetic engineering, plant genetic improvement and other directions, can solve the problems of low yield and poor stability of phospholipase C
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Embodiment 1, cloning and expression of Aspergillus oryzae phospholipase C gene AoPC
[0099] 1. Amplification of gene AoPC
[0100] 1. Extract the RNA of Aspergillus oryzae, reverse transcribe it into cDNA, use the cDNA as a template, and carry out PCR amplification with the following primers:
[0101] Upstream primer: 5'-AAA TACGTA AGCCCTGTCACGTCCGAG-3' (the underline indicates the SnaBI restriction site)
[0102] Downstream primer: 5'-AAA GCGGCCGC TTATACCGAAAAGGTATGGGGAGTCTTG-3' (the underline indicates the NotI restriction site).
[0103] 2. The PCR conditions in step 1 are pre-denaturation at 94°C for 5 minutes; denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, extension at 72°C for 90 seconds, 30 cycles; extension at 72°C for 5 minutes.
[0104] The amplified PCR product has the nucleotide sequence shown in Sequence Table 1 in the Sequence Listing, and the gene indicated by the nucleotide is named Gene AoPC, wherein the 58th-1395th posit...
Embodiment 2
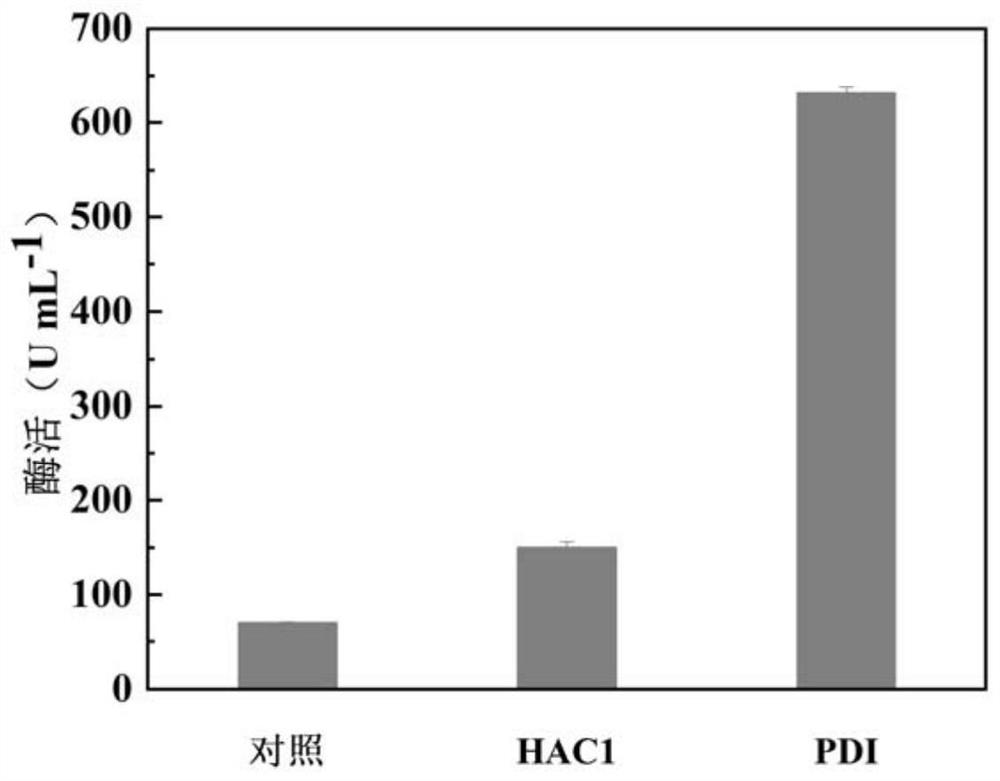
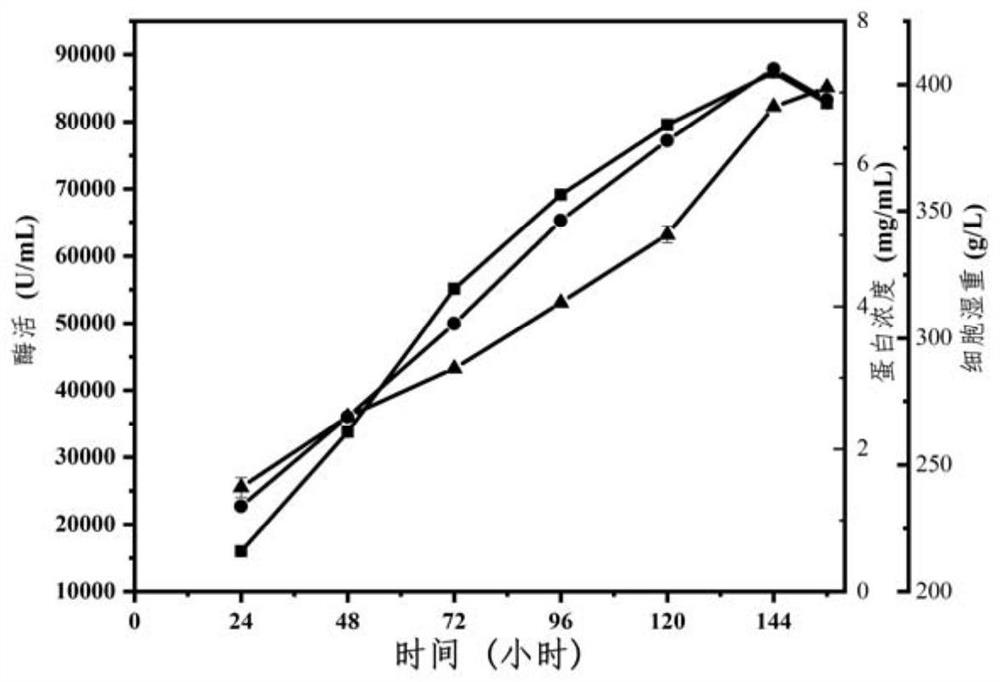
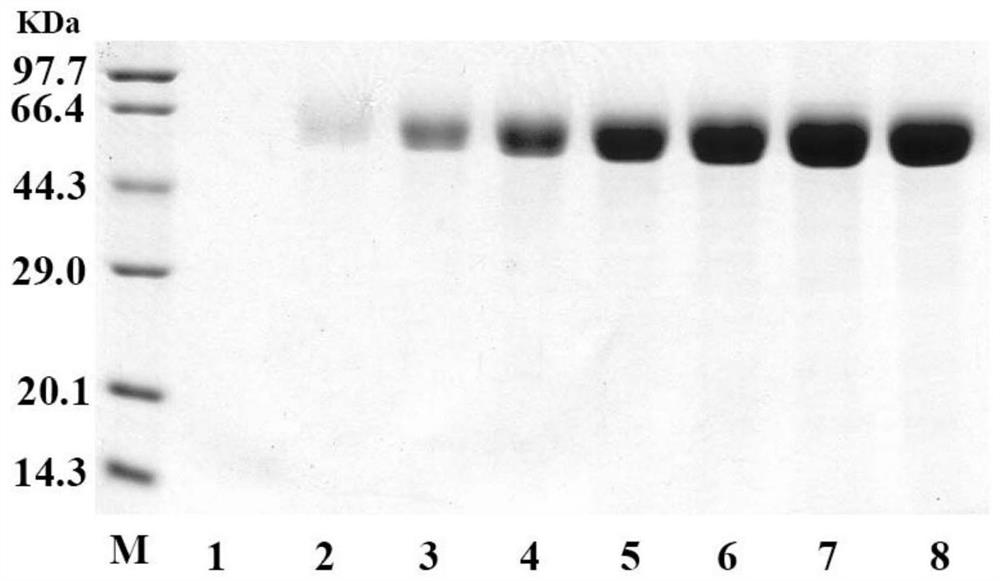
[0147] Example 2, Purification and enzymatic property determination of recombinant phospholipase C AoPC
[0148] 1. Purification of recombinant phospholipase C AoPC
[0149] 1. Dialyze the fermentation supernatant of recombinant bacteria GS115 / pPIC9K-AoPC-PDI with 5L of 20mM Tris-HCl pH 7.5 buffer solution (buffer solution A) for 12h, and incubate at 4° C. , refrigerated and centrifuged at 10,000 rpm for 10 min, and the supernatant was reserved for later use to obtain a crude enzyme solution (concentration: 7.3 mg / mL).
[0150] 2. Purify the crude enzyme liquid obtained in step 1 through a QSFF strong anion exchange column (1.0×10 cm), using an AKTA protein purification system (purchased from GE Healthcare, USA), and the sample loading flow rate is 0.5 mL / min.
[0151] 3. After completing step 2, set the flow rate to 1mL / min, and use buffer solution A to elute to the eluent OD 280 <0.05.
[0152] 4. After completing step 3, use buffer solution A containing 150mM NaCl to elute...
Embodiment 3
[0201] Embodiment 3, the application of recombinant phospholipase C AoPC in soybean crude oil degumming
[0202] 1. Screening of optimal conditions for recombinant phospholipase C AoPC in soybean oil degumming
[0203] 1. Recombinant phospholipase C AoPC phospholipid degumming and acid addition optimization
[0204] AoPC+Crtric acid: Weigh 300g of crude soybean oil (the oil that has not undergone post-degumming treatment) in a 100mL Erlenmeyer flask with a stopper and heat it to 70°C in a water bath, add 0.3mL of citric acid solution (prepared with water with a concentration of 0-50%) Citric acid solution, constant volume, w / w), pretreatment at 200rpm for 20min. After the treatment is completed, cool to the preset temperature (20-50°C, can be 25°C), add 0.6mL of 4% (g:ml) NaOH aqueous solution to adjust the pH to 8, then add 1% distilled water and a certain amount of The 14.8mg / mL recombinant phospholipase C AoPC pure enzyme solution obtained in the first step of the prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com