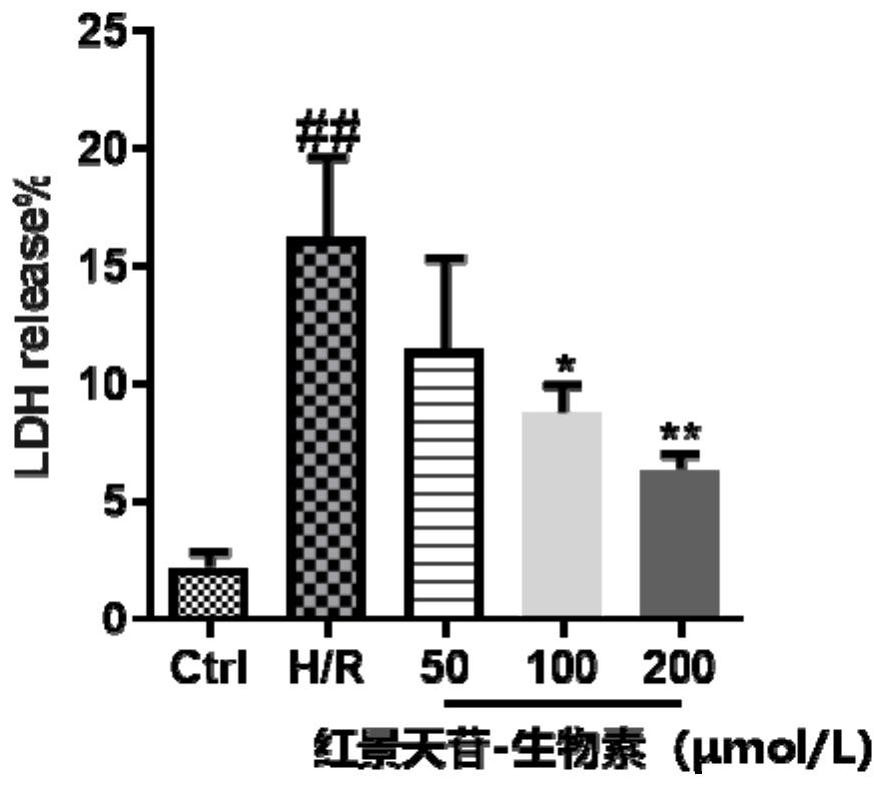
Salidroside-biotin small molecular probe as well as preparation method and application thereof
A technology of small molecule probe and salidroside, which is applied in the field of salidroside-biotin small molecule probe and preparation thereof, and achieves the effects of good therapeutic effect, high selectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of salidroside nitrate (VII)
[0040]
[0041] In a 25 mL round bottom flask, 97 mg of iodobenzene diacetoxyacetate, 142 mg of ferric nitrate nonahydrate and acetonitrile (8 mL) were added, and 300 mg of salidroside (VI) was added. The reaction solution was stirred and reacted at 65°C for 6 hours. The reaction solution was filtered with suction to remove insoluble matter, and then concentrated. The concentrate was subjected to silica gel column chromatography. The eluent used in the chromatography was a mixture of dichloromethane and methanol at a volume ratio of 10:1. The eluate was evaporated to remove the solvent to obtain 245 mg of yellow solid with a yield of 71%.
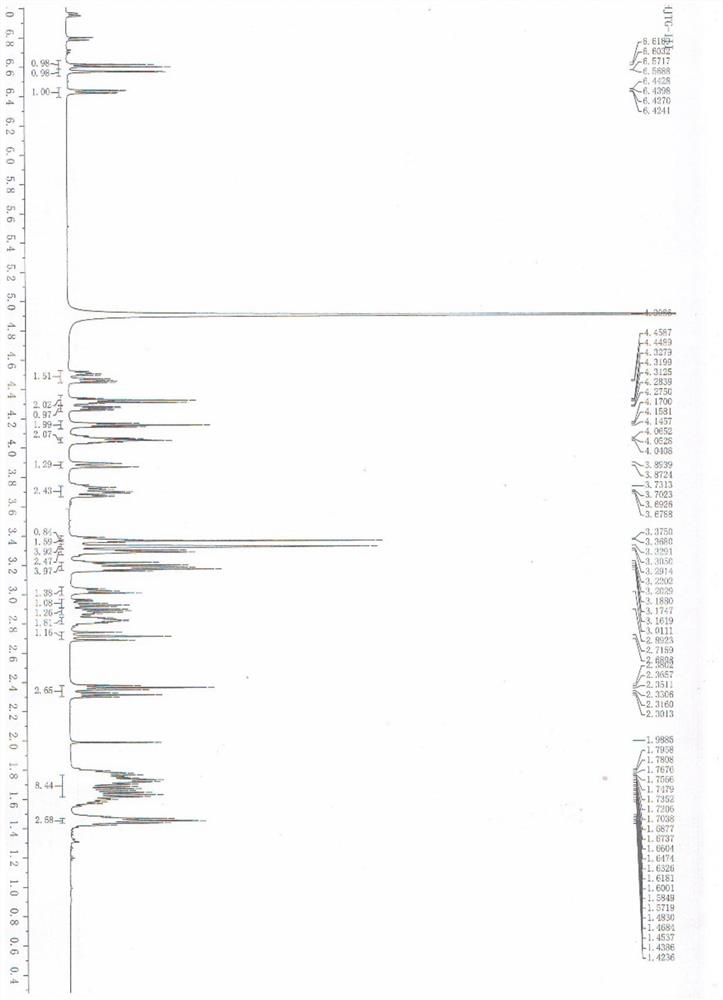
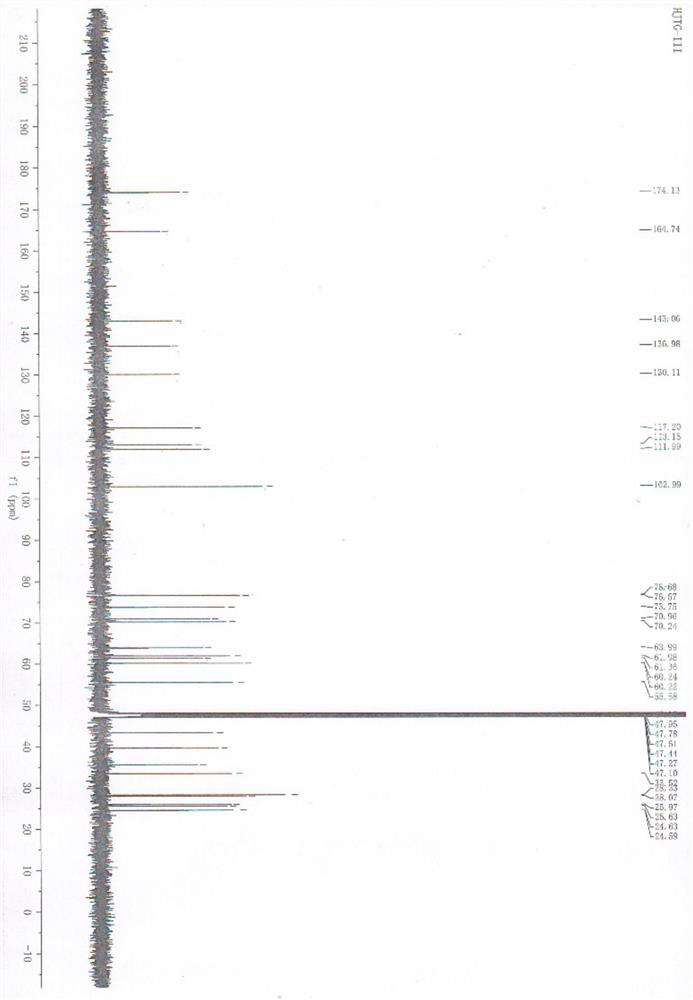
[0042] The spectral data analysis of compound VII is as follows:
[0043] 1 H NMR (CD 3 OD,500MHz)δ2.83(t,J=6.75Hz,2H),3.06-3.09(m,1H),3.10-3.11(m,2H),3.20-3.21(m,1H),3.22-3.27(m ,1H),3.54-3.55(m,1H),3.65-3.69(m,1H),3.75-3.78(m,1H),3.91-4.03(m,1H),4.19(d,J=7.8Hz,1H ...
Embodiment 2
[0046] Embodiment 2: the preparation of salidroside nitrate (VII)
[0047]In a 25 mL round bottom flask, 118 mg of bis(tert-butylcarbonyloxy)iodobenzene, 131 mg of ferric nitrate nonahydrate and acetonitrile (8 mL) were added, and 290 mg of salidroside were added. The reaction solution was stirred and reacted at 55°C for 10 h, the reaction solution was filtered with suction to remove insoluble matter, and then concentrated, and the concentrate was subjected to silica gel column chromatography. The eluent used in the chromatography was a mixture of dichloromethane and methanol at a volume ratio of 10:1. The eluate was evaporated to remove the solvent to obtain 219 mg of a yellow solid with a yield of 66%.
Embodiment 3
[0048] Embodiment 3: the preparation of salidroside nitrate (VII)
[0049] In a 25 mL round bottom flask, 134 mg of [bis(trifluoroacetoxy)iodo]benzene, 148 mg of ferric nitrate nonahydrate and acetonitrile (8 mL) were added, and 310 mg of salidroside were added. The reaction solution was stirred and reacted at 50°C for 6 hours. The reaction solution was filtered to remove insoluble matter, and then concentrated. The concentrate was subjected to silica gel column chromatography. The eluent used in the chromatography was a mixture of dichloromethane and methanol at a volume ratio of 10:1. The eluate was evaporated to remove the solvent to obtain 306 mg of a yellow solid with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com