A kind of biological preparation method of caffeic acid
An amino acid and tryptophan technology, applied in the field of microorganisms, can solve problems such as affecting food safety and affecting the application prospect of caffeic acid, and achieve the effects of high conversion rate and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
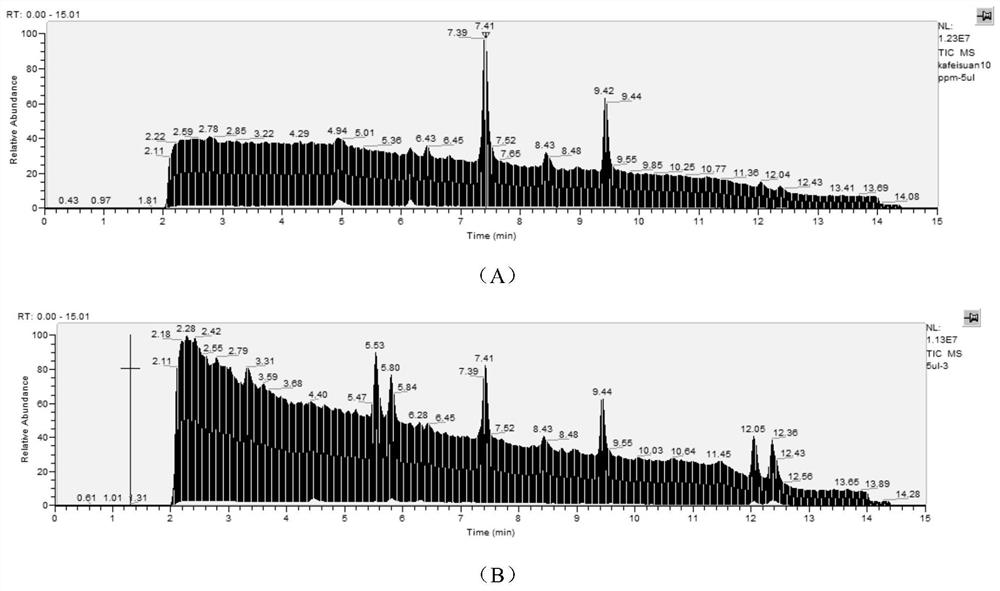
Image
Examples
Embodiment 1
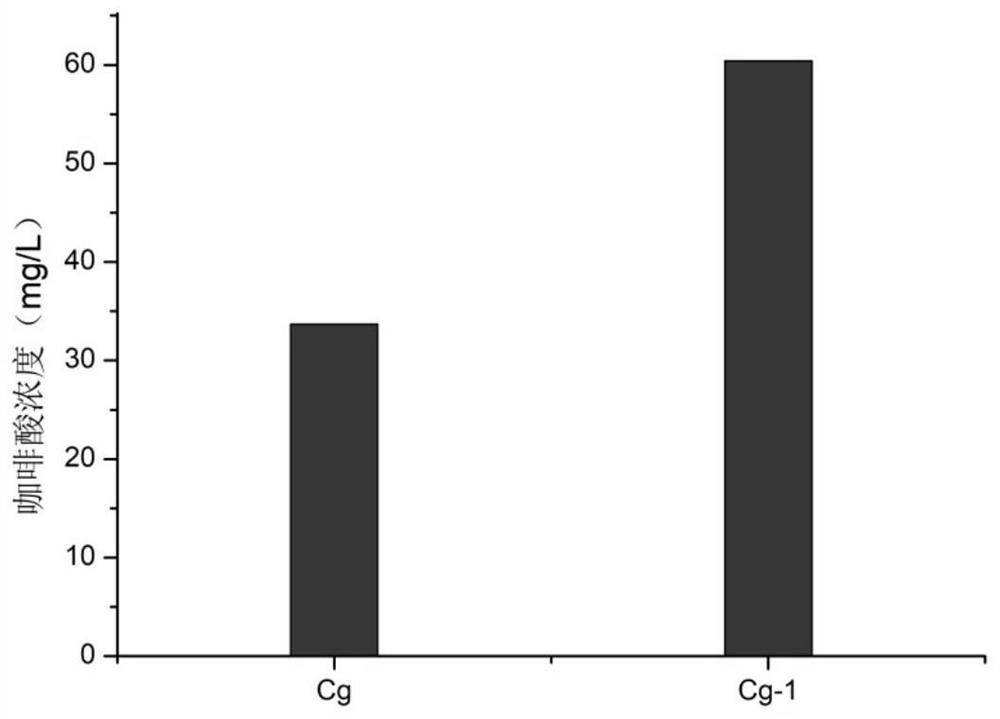
[0032] Example 1 Construction of recombinant Candida glycerologenus Cg-1 producing caffeic acid
[0033] Construction of recombinant bacteria: using Candida glycerinogenes CCTCC M 93018 as the starting strain, using CRISPR-Cas9 technology to knock out and knock in genes, by knocking out L-tryptophan shown in SEQ ID NO.7 Synthetic gene trp1 and the L-phenylalanine synthetic gene pheA shown in SEQ ID NO.8 knock out the competing pathway L-tryptophan and L-phenylalanine in the starting strain for L-tyrosine synthesis; Overexpression of the 3-deoxy-D-arabinoheptonate-7-phosphate synthase gene aro4 shown in SEQ ID NO.9 and the bifunctional choroidal acid synthase and flavin reductase genes shown in SEQ ID NO.10 aro7 releases the feedback inhibition effect of L-tyrosine, and transforms into the production host Cg-1.
Embodiment 2
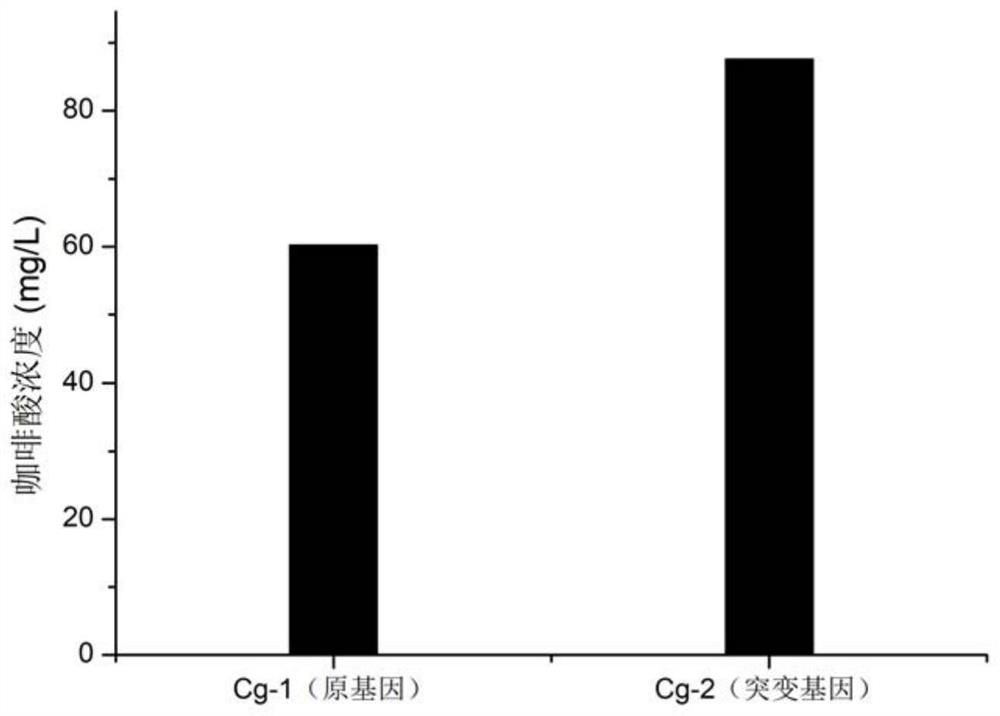
[0035] Gene mutation: According to the software analysis of the protein structure of the three genes of tyrosine ammonia lyase FjTAL, 4-hydroxyphenylacetic acid-3-monooxygenase HpaB and NADPH flavin oxidoreductase HpaC, Leu151 of FjTAL and Ala195 of HpaB were found Form a hydrogen bond with the substrate, and analyze the possible combination with the substrate and the important role of glycosidic bond breaking. Therefore, saturation mutations were performed on the coding genes of the above three enzymes to increase the conversion rate. After fusion expression of the mutant genes, it was found that only by mutating the leucine Leu at position 151 of FjTAL to glycine Gly, and the alanine Ala at position 195 of HpaB to serine Ser, the production of caffeic acid was significantly improved. The gene loses its activity or the yield decreases, and the mutated fusion gene is expressed in the production host Cg-1 to obtain the bacterial strain Cg-2. Compared with the unmutated engineeri...
Embodiment 3
[0036] Example 3 Construction of a linearized plasmid expressing a fusion gene
[0037]Linearized vector preparation: The fusion gene FjTAL-HpaB-HpaC was obtained by tandem encoding mutant enzyme genes FjTAL, HpaB and HpaC using the connecting peptide GSG, digested with restriction endonucleases BamHI and NotI, and connected to the same enzyme A recombinant expression vector was constructed on the excised pURGAPU plasmid (SEQ ID NO.11), and SacI was used to linearize the recombinant expression vector.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com