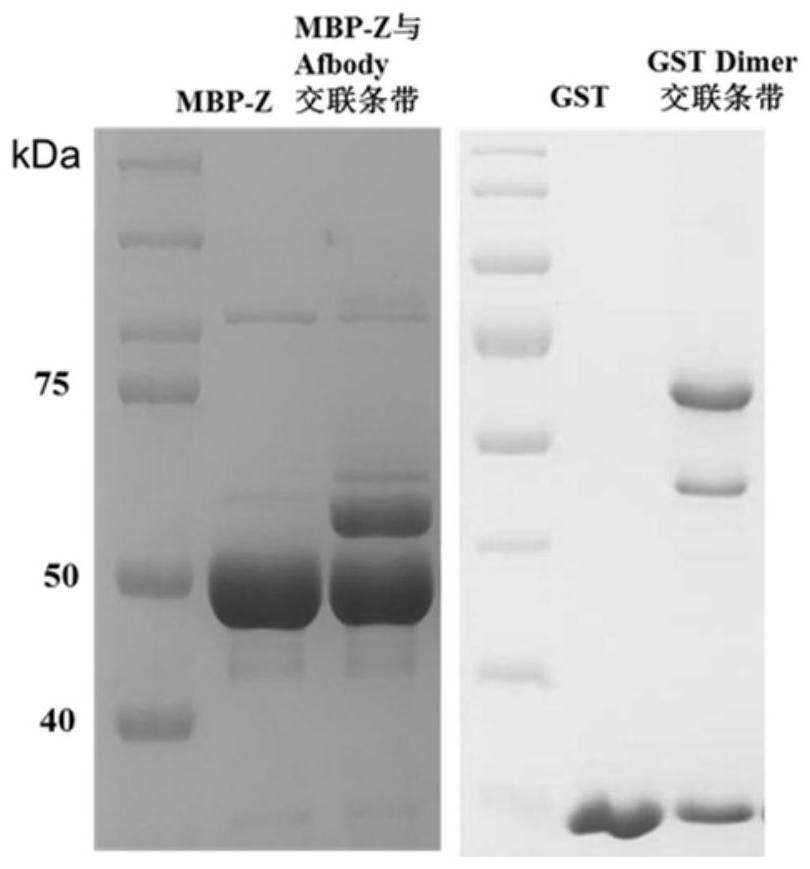
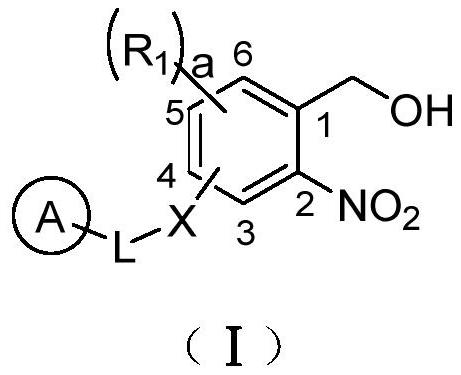
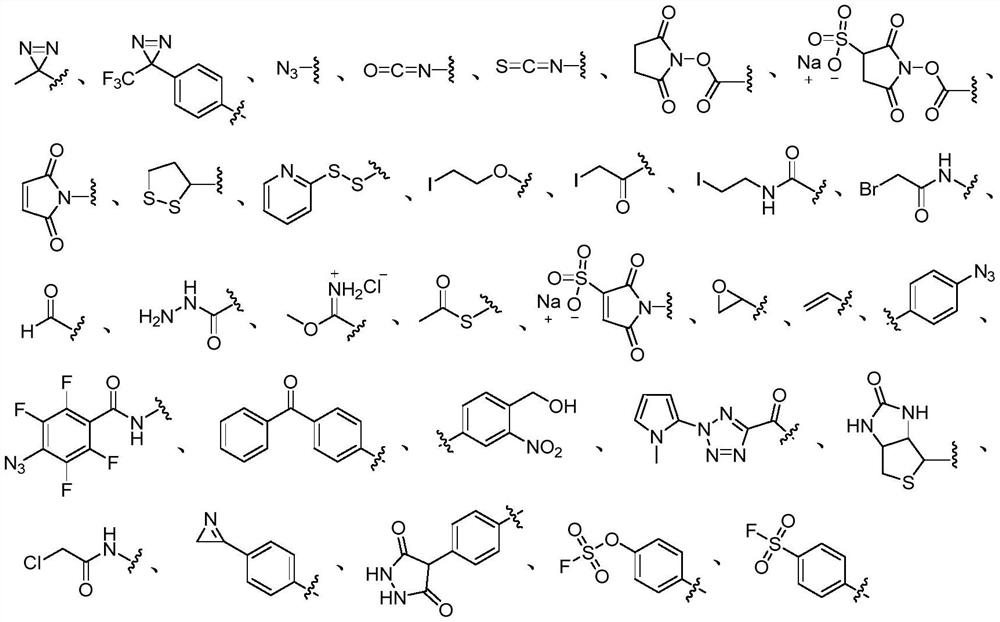
Photo-induced multifunctional cross-linking agent, preparation method and application thereof
A compound and selected technology, applied in the field of protein cross-linking and protein analysis research, can solve the problems of mass spectrometry analysis and protein interaction confirmation, difficulty in synthesis of structural specificity, complex structure of cross-linked peptides, etc. Reaction efficiency, good ability to capture interacting proteins, effect of good reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 6
[0155] Example 6: N-(6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexyl)-4-(hydroxymethyl)-3-nitrobenzyl Amide
[0156]
[0157] Step 1-1: Dissolve 4-bromomethyl-3-nitrobenzoic acid (5.0 g, 19.31 mmol, 1.0 eq) in 60 mL of acetone, add 60 mL of water, and stir well. Anhydrous sodium carbonate (7.16 g, 67.58 mmol, 3.5 eq) was then added. The resulting mixture was reacted at 70°C for 2 hours. The reaction was monitored by LC-MS. After the reaction was completed, the pH was adjusted to 3-4 with 2N hydrochloric acid, extracted with ethyl acetate, dried, and the solvent was spun off to obtain 4.02 g of compound 1D as a yellow solid, which was directly injected into the next step without further purification. 1 H NMR (400MHz, CDCl 3 )δ8.79(d, J=1.6Hz, 1H), 8.36(dd, J=8.1, 1.7Hz, 1H), 7.96(d, J=8.2Hz, 1H), 5.11(s, 2H).
[0158] Step 1-2: Compound 1A (54 mg, 0.275 mmol) was added to 2:1 CH 2 Cl 2 and trifluoroacetic acid (1.5 mL), stirred at room temperature for 1 h and the solven...
Embodiment 223
[0160] Example 223: N-(6-(2-Bromoacetamido)hexyl)-4-(hydroxymethyl)-3-nitrobenzamide
[0161]
[0162] Step 2-1: Dissolve 2-bromoacetic acid (2.00g, 14.39mmol) in anhydrous 1,4-dioxane (15mL), add NHS (1.66g, 14.39mmol), dropwise at 0°C A solution of DCC (3.27 g, 15.83 mmol) in anhydrous 1,4-dioxane (5 mL), the reaction mixture was stirred at room temperature for 2 h and filtered, the filtrate was concentrated in vacuo to give compound 2A as a yellow oil (2.43 g, 77% ). 1 H NMR (400MHz, DMSO) δ4.64(s, 2H), 2.83(s, 4H).
[0163]Step 2-2: Compound 1D (400 mg, 2.03 mmol), HATU (849 mg, 2.23 mmol) and tert-butyl(6-aminohexyl) carbamate (483 mg, 2.23 mmol) were added in dry DMF (10 mL) DIPEA (1 mL, 6.09 mmol) was added dropwise at 0°C, and the mixture was stirred at room temperature overnight. Add H to the mixture 2 O, and extracted 3 times with ethyl acetate. The organic layer was washed with water, brine, dried over sodium sulfate and concentrated in vacuo. The residue w...
Embodiment 229
[0165] Example 229: 4-(2-(4-(Hydroxymethyl)-3-nitrobenzamido)ethyl)phenylfluorothioate
[0166]
[0167] Step 3-1: Compound 1D (1.00 g, 5.07 mmol) and NHS (702 mg, 6.08 mmol) were dissolved in DMF (15 mL), EDCI·HCl (1.16 g, 6.08 mmol) was added, and the reaction mixture was stirred at room temperature for 2 h. Add H to the mixture 2 O, and extracted 3 times with ethyl acetate. Organic layer with 2.8% KHSO 4 , washed with brine, washed with anhydrous Na 2 SO 4 Dry and concentrate in vacuo. The residue was purified by silica chromatography to give the product Compound 3A (710 mg, 48%) as a yellow oil.
[0168] Step 3-2: Fill chamber A of a dry two-chamber reaction flask with 1,1'-sulfonimidoimidazole (SDI, 1.487g, 7.5mmol) and potassium fluoride (KF, 1.162g, 20.0mmol) . Next, compound 3B (1.18 g, 5.0 mmol), triethylamine (1.53 mL, 11.0 mmol) and dichloromethane (DCM, 4 mL) were added in chamber B. Finally, 15 mL of a trifluoroacetic acid (TFA) / water mixture was inject...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com