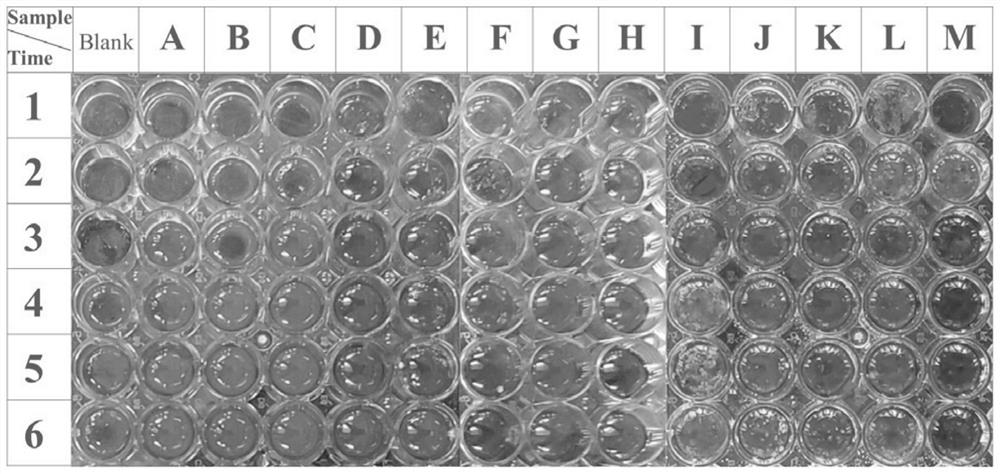
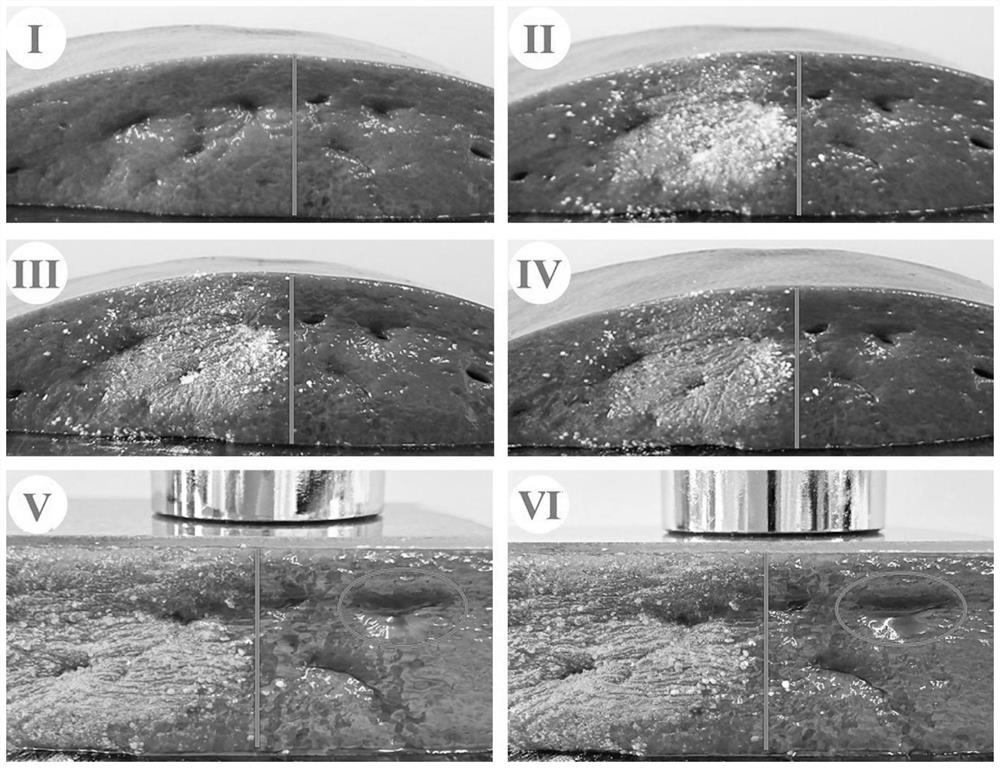
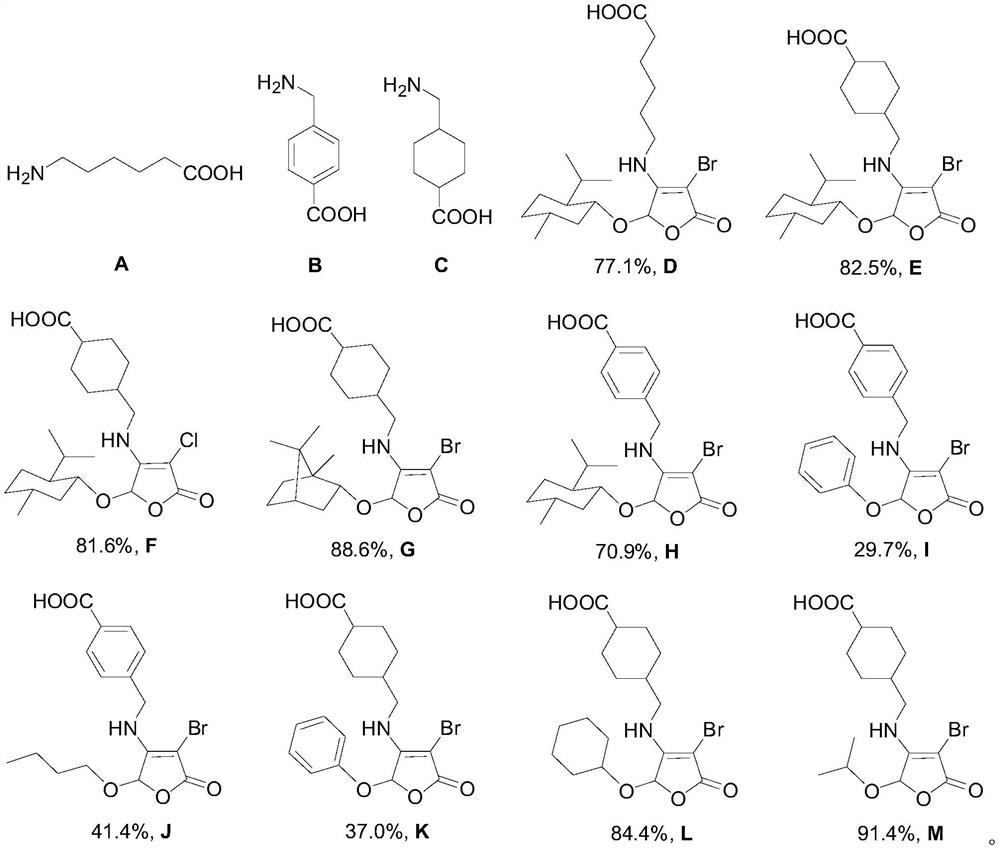
Furanone-based amino acid compound, preparation method thereof and application of furanone-based amino acid compound as coagulant drug
A furanone-based amino acid and compound technology, which is applied in the field of furanone-based amino acid compounds and their preparation, can solve the problems of single and oligomeric types of functional groups, simple molecular structure, limited clinical application and the like, and achieves short coagulation time and preparation method. Simple, easy-to-use post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 5-Mengli-3-bromo-2 (5H) furanone-based amino acid (compound D) synthesis:
[0035] 0.3961 g (2.00 mmol) of aminocyanoic acid and 0.1257 g (2.24 mmol) of potassium hydroxide were added to 50 ml of the two-necked carcolor, and then 5 ml of anhydrous ethanol was added, stirred and dissolved, and the vacuum excavated Air, filled with nitrogen protection, stirred, and then 0.3961 g (1 mmol) of 5-Mengli-3-bromo-2 (5H) furanone is dissolved with 5 mL of dichloromethane by constant pressure droplet funnel. In the dual-necked flask, 1 drop of 0.0 min is added, 30 min is added, stirred at room temperature for 24 h, and the reaction liquid is adjusted by the reaction liquid by 15% hydrochloric acid after the reaction, and extract 3 times with ethyl acetate (20 ml each time Ethyl acetate, separation, dry dry with anhydrous sodium sulfate, the crude product is separated from the column chromatography, resulting in 0.3442 g of 5-Mengli-3-bromo-2 (5H) furanone-ketoic acid (light Yellow so...
Embodiment 2
[0040] 5-Mengli-3-bromine-2 (5H) furanone-based amine acid (compound E) synthesis:
[0041] 0.1570 g (1.00 mmol) of aminethylcyclo acid and 0.1042 g (1.12 mmol) of potassium hydroxide were added to 50 ml of the bicycle, and then 7 ml of anhydrous ethanol was added, stirred and dissolved, and the vacuum was exhausted. Air, filled with nitrogen, stirred, and then 0.1980 g (0.50 mmol) of 5-Mengli-3,4-dibromo-2 (5H) furanone is dissolved after 5 ml of dichloromethane. Drip funnel is added to the di-neck flask, 1 drop per 60s, 20 min, at room temperature for 28 h, and the reaction liquid is adjusted with a mass fraction of 15% hydrochloric acid, extracts 3 times with ethyl acetate (EtOAc EtOAc) EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc Aminochlorocyathic acid (white solid, melting point 115.3 ~ 116.5 ° C, yield 82.5%).
[0042] 5-Mengli-3-bromine-2 (5H) furanone-based ammonium methic acid's nuclear magnetic resonance spectrum data is as follows:
[0043] 1H NMR (150MHz, CDC...
Embodiment 3
[0050] 5-Mengli-3-chloro-2 (5H) furanone-based amine acid (compound F) synthesis:
[0051] 0.10 59 g (0.674 mmol) of aminethoxyic acid and 0.0424 g (0.7549 mmol) of potassium hydroxide were added to 50 ml of the secondary and neculic flask, and then 5 ml of anhydrous ethanol was added, stirred and dissolved, and the vacuum was exhausted. Air, filled with nitrogen, stirred, and then 0.1034 g (0.337 mmol) 5-Mengli-3,4-dichloro-2 (5H) furanone is dissolved after 5 ml of dichloromethane. Drip funnel is added to the di-neck flask, 1 drop per 60s, 20 min, at room temperature for 28 h, and the reaction liquid is adjusted with a mass fraction of 15% hydrochloric acid, extracts 3 times with ethyl acetate (EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc Aminochlorocyathic acid (white solid, melting point 108.4 ~ 109.7 ° C, yield 81.6%).
[0052] The nuclear magnetic resonance spectrum data of 5-Mengli-3-chloro-2 (5H) furanone-based amine acid is as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com