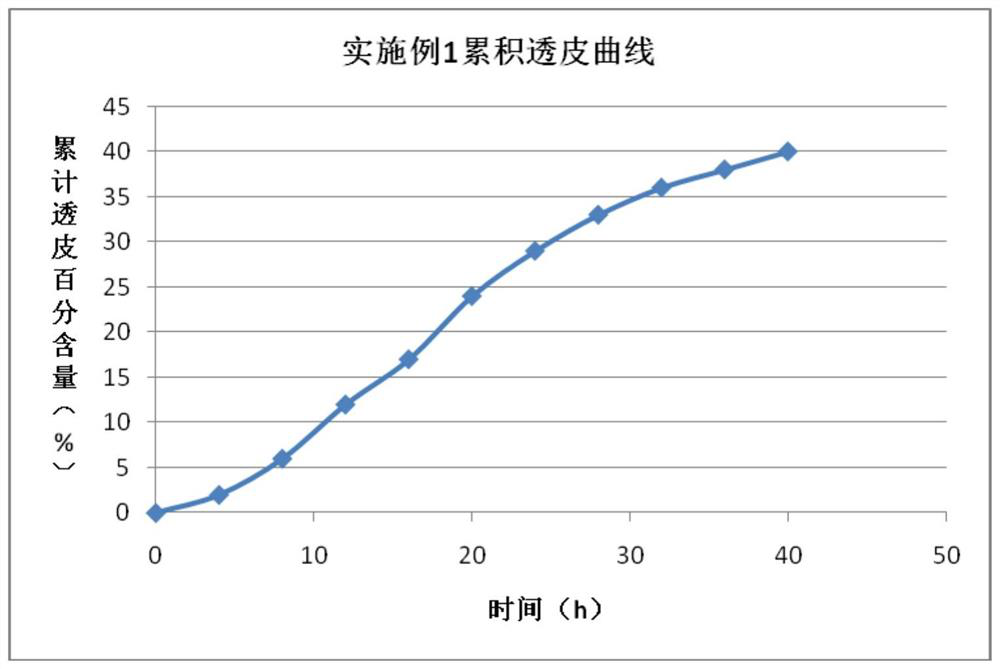
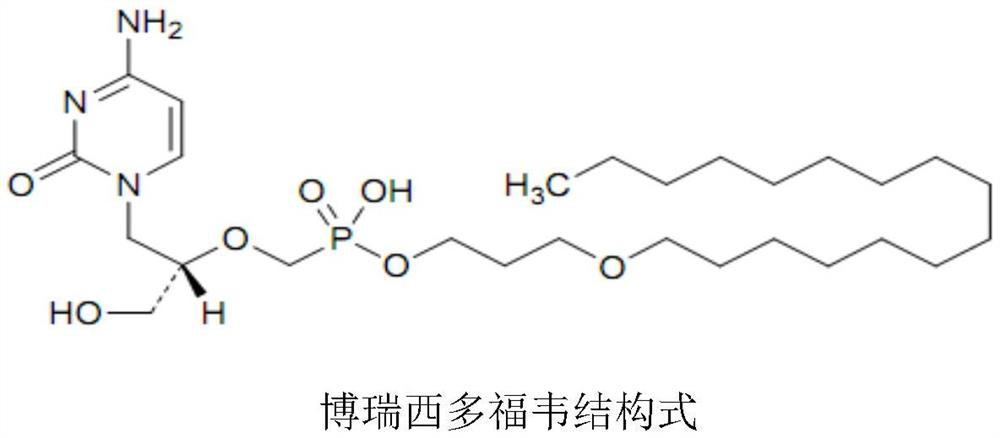
Borasidofovir cream
A technology of boricidofovir and emulsifier, applied in the directions of antiviral agent, ointment delivery, aerosol delivery, etc., can solve the problem of no nephrotoxicity or myelosuppression events, achieve good clinical application prospects, improve Safety, efficacy to avoid systemic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
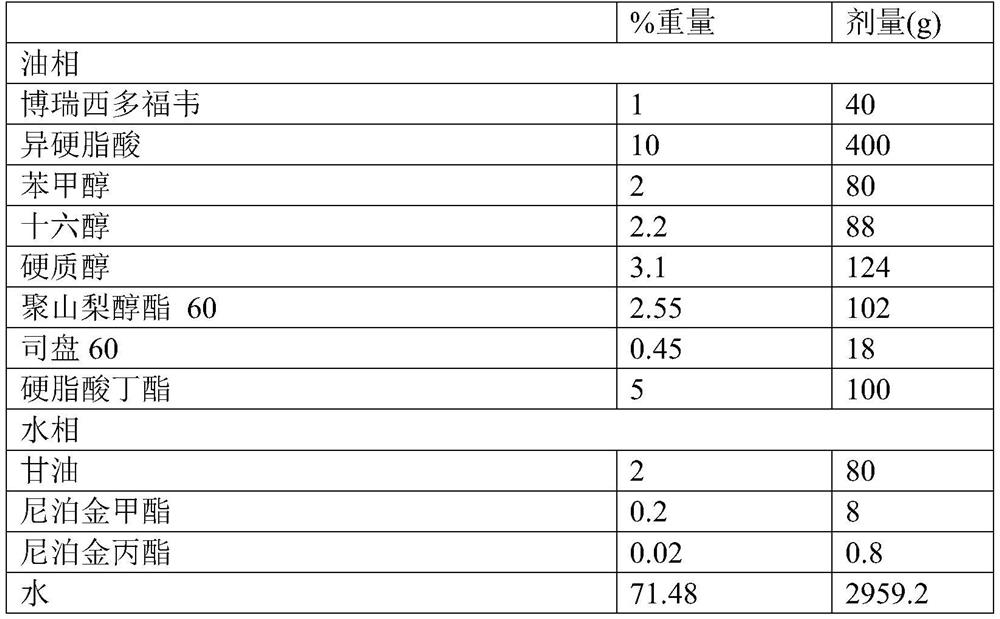
[0034] Prescription listed in table 1 and following process method prepare brecidofovir cream:
[0035] Table 1 Recidofovir cream prescription of 4000g of the present invention as example:
[0036]
[0037] Preparation method: weigh the glycerin, methylparaben, propylparaben and water of the prescription amount in Table 1 and put them in a 4-liter beaker, heat and stir the mixture until the paraben is dissolved; Fovir and isostearic acid were weighed and placed in an 8-liter beaker, heated and stirred until brecidofovir was dissolved (temperature reached 69°C), and benzyl alcohol, cetyl alcohol, stearyl alcohol, polysorbate 60 , Span 60, butyl stearate, weighed and added to the isostearic acid solution, continue to heat and stir until all materials are dissolved (temperature reaches 75°C); at the same temperature (65-75°C), the water phase Add it into the oil phase to mix the two phases. After the resulting mixture is mixed and homogenized for 13 minutes, the container is ...
Embodiment 2-9
[0039] According to the prescription in Table 2, the general preparation process method described in Example 1 is used to prepare brecidofovir cream:
[0040] Table 2 Prescription of brecidofovir cream
[0041]
Embodiment 10
[0043] Prescription listed in table 3 and the following process method take 300g as an example to prepare brecidofovir cream:
[0044] Table 3 Prescription of brecidofovir cream
[0045]
[0046] Preparation method: weigh methylparaben and water according to the prescription amount and put them in a beaker, heat and stir the mixture until the paraben is dissolved (the temperature reaches 60-65°C), add magnesium aluminum silicate colloid, and dissolve the obtained aqueous solution at 75 Heat and stir at ℃ for 30 minutes to obtain a uniform solution; take another brecidofovir and isostearic acid and put them in a beaker after weighing, heat and stir until brecidofovir dissolves (the temperature reaches 68 ℃), then add white Petroleum jelly, light liquid paraffin, aluminum stearate, cetyl alcohol, glyceryl oleate, acetyl lanolin and propylparaben, the resulting oil phase mixture is heated to 75°C and mixed evenly. The two phases obtained are at the same temperature (75°C), ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com