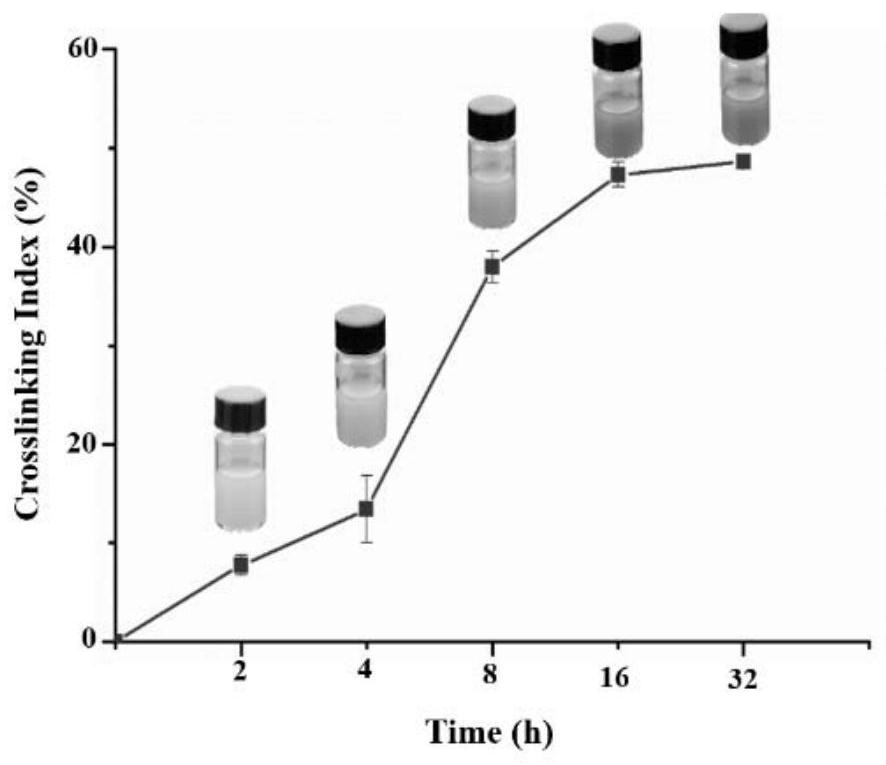
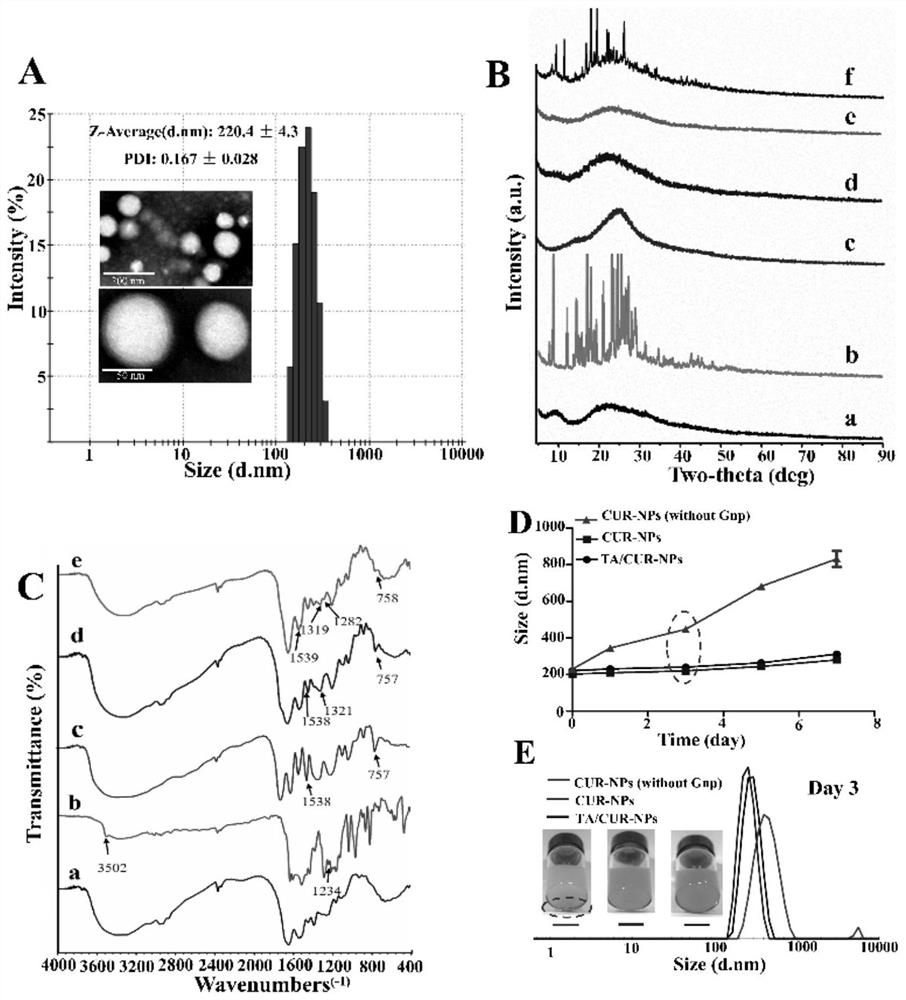
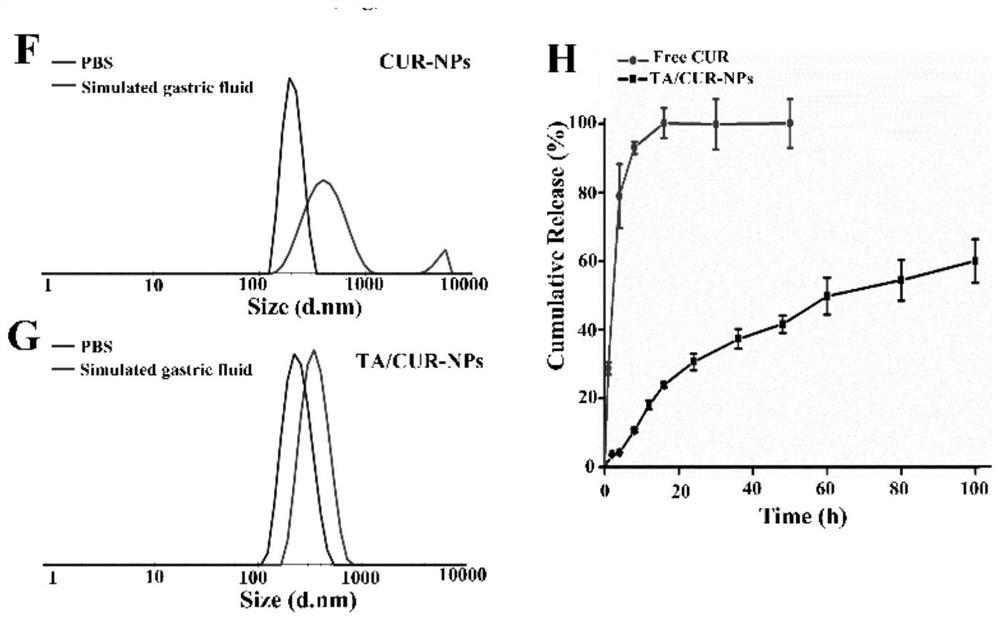
Tannic acid curcumin nanoparticles and preparation method and use thereof
A nanoparticle and tannic acid technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, non-effective ingredients of polymer compounds, etc., can solve problems such as toxicity, and achieve improvement of colitis, good adhesion, and particle size evenly distributed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1, the preparation of tannic acid curcumin nanoparticles of the present invention
[0058] The present invention mainly adopts oil-in-water (O / W) single emulsion-solvent evaporation technology to prepare tannic acid curcumin nanoparticles (TA / CUR-NPs), and the specific method is as follows:
[0059] (1) Preparation of curcumin nanoparticles: 100mg human serum albumin (HSA) was dissolved in 50mL deionized water to prepare HSA solution; meanwhile, 20mg genipin (Gnp) and 5mg curcumin (CUR) were completely dissolved in 1mL of acetone to obtain the organic phase; the above two organic phases were dropped into the HSA solution under constant magnetic stirring; then the mixture was placed in an ice bath and ultrasonically treated with a probe sonicator at 70% amplitude power for 6 minutes. Forms an oil-in-water emulsion. The emulsion was further stirred at room temperature at 400 rpm for 6 hours to eliminate residual acetone. The emulsion was then introduced into ...
Embodiment 2
[0062] Embodiment 2, the preparation of emodin tannic acid nanoparticles of the present invention
[0063] The present invention mainly adopts oil-in-water (O / W) single emulsion-solvent evaporation technology to prepare emodin tannic acid nanoparticles (TA / EM-NPs), and the specific method is as follows:
[0064] (1) Preparation of emodin nanoparticles: 100 mg of human serum albumin (HSA) was dissolved in 50 mL of deionized water to prepare HSA solution; meanwhile, 20 mg of genipin (Gnp) and 10 mg of emodin (EM) were completely dissolved in 1mL of acetone to obtain the organic phase; the above two organic phases were dropped into the HSA solution under constant magnetic stirring; then the mixture was placed in an ice bath and ultrasonically treated with a probe sonicator at 70% amplitude power for 6 minutes. Forms an oil-in-water emulsion. The emulsion was further stirred at room temperature at 400 rpm for 6 hours to eliminate residual acetone. The emulsion was then introduce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com