Pharmaceutical composition containing erigeron breviscapus extract and borneol and preparation method of pharmaceutical composition
A technology of scutellaria scutellaria and extracts, which is applied in the field of pharmaceutical preparations, can solve the problems of excessively fast release, low in vivo utilization, and failure to meet the requirements of sustained release, and achieve excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Prescription 1
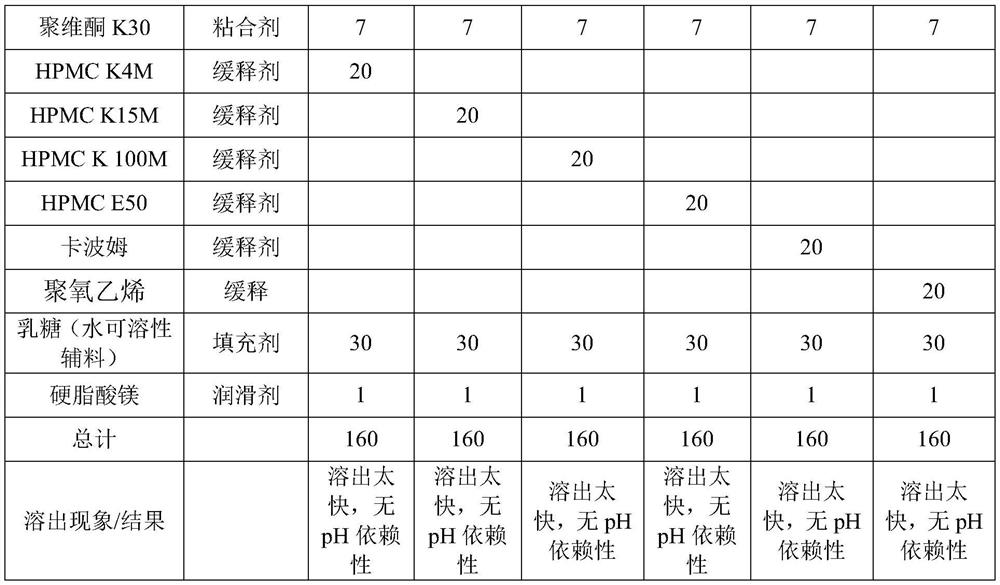
[0059] components effect prescription Erigeron extract API 100 ice flakes stabilizer 2 PVP K30 Adhesive 7 Calcium hydrogen phosphate (Hunan Jiudian Pharmaceutical Co., Ltd.) contains 5% NaHCO3 filler 20 HPMC K4M sustained release agent 30 Magnesium stearate (Shanghai Yunhong Chemical Preparations and Excipients Technology Co., Ltd.) lubricant 1 total 160
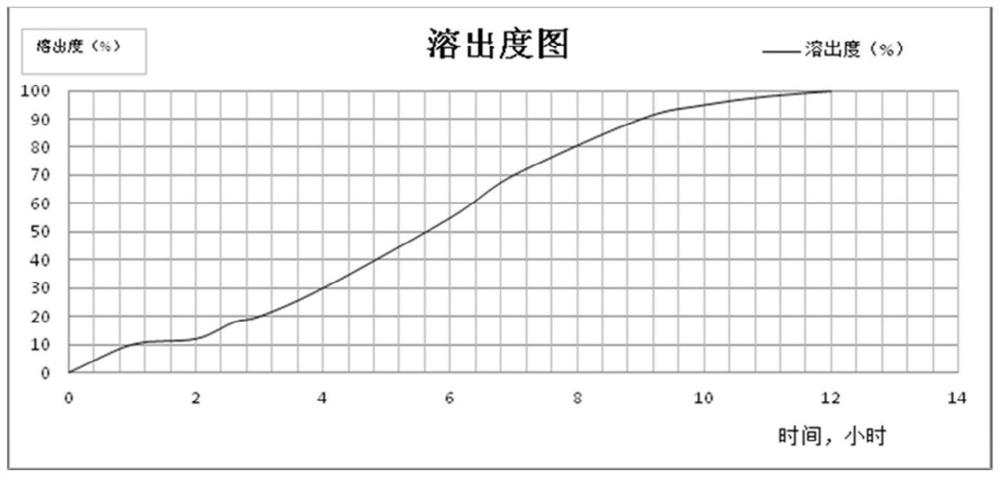
[0060] Preparation method: 1) Mix the raw material drug, binder, filler, and slow-release material evenly, add purified water (the amount is adjusted according to actual operation) for wet granulation, and dry in a fluidized bed until the LODfigure 1 , using the pH simulated release method to obtain an ideal S-type pH-dependent release curve. )
Embodiment 2
[0062] Prescription 2
[0063]
[0064]
[0065] Preparation method: 1) Mix the raw material drug, binder, filler, and slow-release material evenly, add purified water (the amount is adjusted according to actual operation) for wet granulation, and dry in a fluidized bed until the LOD<1.0%. After granulation by 1016μm Comil, add lubricant, mix evenly, and perform tablet compression, the theoretical tablet weight is 1000mg; tablet hardness is controlled at 150N.
Embodiment 3
[0067] Prescription 3
[0068] components effect prescription Erigeron extract API 100 ice flakes stabilizer 2 PVP K30 Adhesive 7 Dibasic calcium phosphate anhydrous (Hunan Jiudian Pharmaceutical Co., Ltd.) contains 5% KHCO3 filler 20 HPMC K15M sustained release agent 30 silica lubricant 1 total 160
[0069] Preparation method: 1) Mix the raw material drug, binder, filler, and slow-release material evenly, add purified water (the amount is adjusted according to actual operation) for wet granulation, and dry in a fluidized bed until the LOD<1.0%. After granulation by 1016μm Comil, add lubricant, mix evenly, and perform tablet compression, the theoretical tablet weight is 1000mg; tablet hardness is controlled at 150N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com