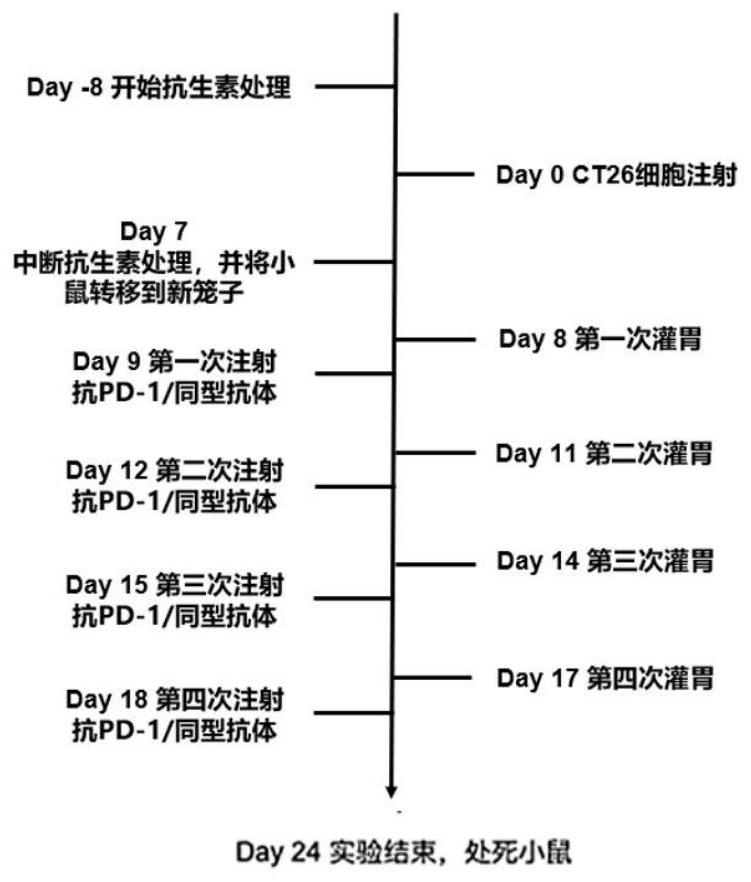
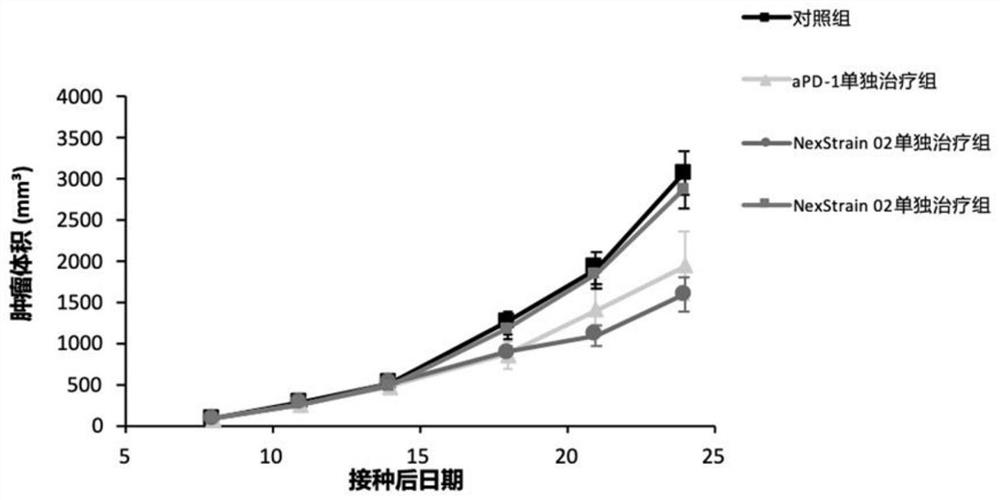
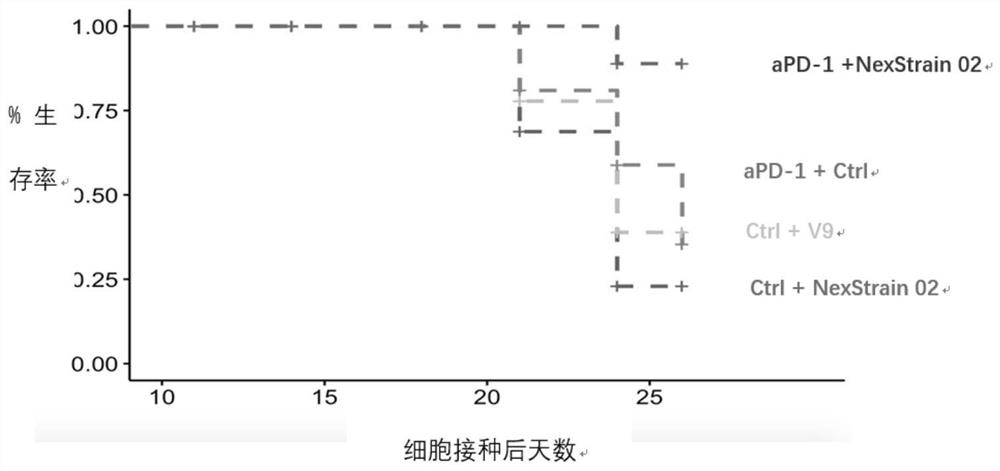
Application of bifidobacterium animalis in improving tumor immunotherapy response
A technology for animal bifidobacterium and tumor treatment, applied in the field of biomedicine, can solve the problems of early termination of treatment, side effects, high price, etc., and achieve the effects of improving immunotherapy response, prolonging survival time, and simple treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1: Bifidobacterium animalis subsp.Lactis alone or in combination with PD-1 immunosuppressant for the treatment of gastrointestinal tumors
[0108] 1.1 Materials, instruments and sources
[0109] Test drug:
[0110] Bifidobacterium animalis NexStrain 02 (deposit number: CGMCC No.20455)
[0111] Packing: 20g / pack x 1 pack, 1x 10 11 CFU / g
[0112] Storage temperature: 4°C
[0113] Isotype Control Antibody Rat IgG2a
[0114] Supplier: Sino-US Crown Biotechnology Co., Ltd.
[0115] Item No.: CVP039
[0116] Storage temperature: 4°C
[0117] aPD-1 antibody (RMP1-14)
[0118] Supplier: Sino-US Crown Biotechnology Co., Ltd.
[0119] Item No.: CVP033
[0120] Storage temperature: 4°C
[0121] Antibodies for Flow Cytometry (Table 1)
[0122] Table 1. Antibody information used in flow cytometry analysis
[0123]
[0124] Reagent:
[0125] Normal saline; supplier: Anhui Double Crane Pharmaceutical Co., Ltd.; batch number: 171005
[0126] RMPI1640; Supplier...
Embodiment 2
[0179] Embodiment 2: flow analysis (FACS)
[0180] On the 16th day after administration (24th day after cell inoculation), 5 tumors in each group were taken for FACS. The harvested tumor samples were digested into a single cell suspension, then added with BFA (bovine calf serum) for blocking, cultured for 4 hours and then harvested for staining. The data obtained after flow cytometry was applied to the computer was analyzed by Kaluza software (Table 6, Figure 4-7 ), all tests were two-tailed, and a p-value less than 0.05 was considered statistically significant.
[0181] Table 6. Results of flow cytometry analysis
[0182]
[0183] Note: P values less than 0.05 are marked with a gray background
[0184]It can be seen from the above that the NexStrain 02 combined treatment group has significant differences compared with the control group in the following indicators: CD4+CD62L-CD44+, CD8+IFN-gamma+, CD4+TNF-alpha+ and Cr-1+CD86+. And the NexStrain 02+aPD- treatment gro...
Embodiment 3
[0185] Example 3: Bifidobacterium animalis subsp.Lactis administered simultaneously or in advance, alone or in combination with PD-1 immunosuppressant, for the treatment of gastrointestinal tumors
[0186] 3.1 Materials, instruments and sources
[0187] Experimental animals: BALB / c mice, female, 7-8 weeks old (the age of mice at the time of tumor cell inoculation), weighing 16.5-22.3 g, 84 mice. Purchased from Shanghai Lingchang Biotechnology Co., Ltd., animal certificate number: 20180003007017. Breeding environment: SPF grade.
[0188] The environmental conditions of the experimental animal breeding room: the experimental animals are all kept in an independent ventilation box with constant temperature and humidity, the temperature of the breeding room is 21-25°C, the humidity is 43-70%, the ventilation is 10-20 times / hour, and the light and dark alternate time of day and night is 12h / 12h; Cobalt 60 radiation sterilized rats were continuously supplied with full-price pellet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com