Catalyst for producing low-carbon olefin, preparation method and application thereof
A low-carbon olefin and catalyst technology, which is applied in the field of catalysts for the direct production of low-carbon olefins by Fischer-Tropsch synthesis, can solve the problems of low selectivity of low-carbon olefins and the like, and achieve the effects of high conversion rate of raw materials and improved catalytic performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
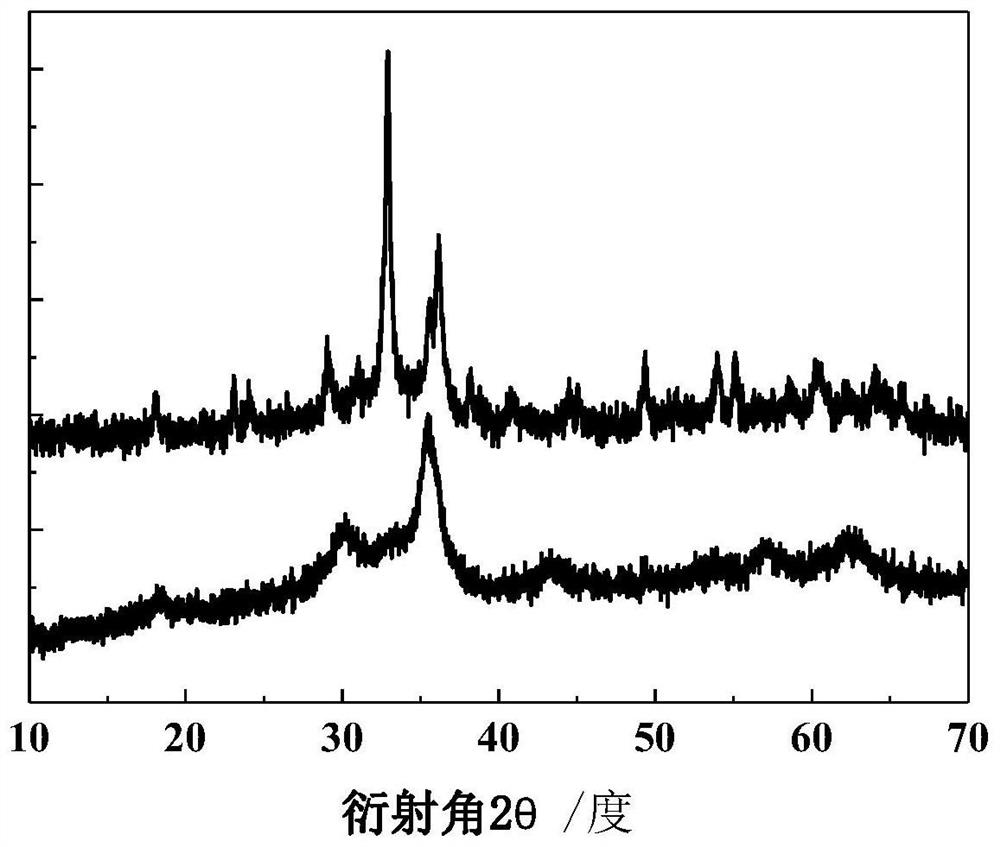
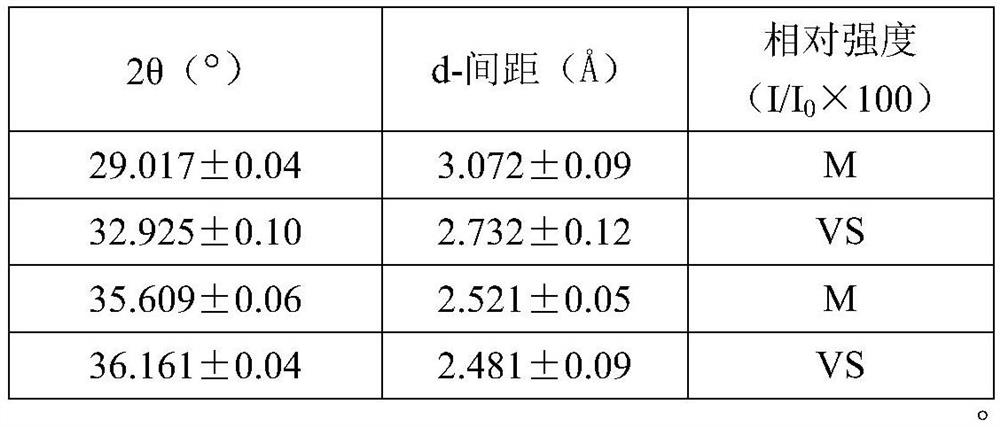
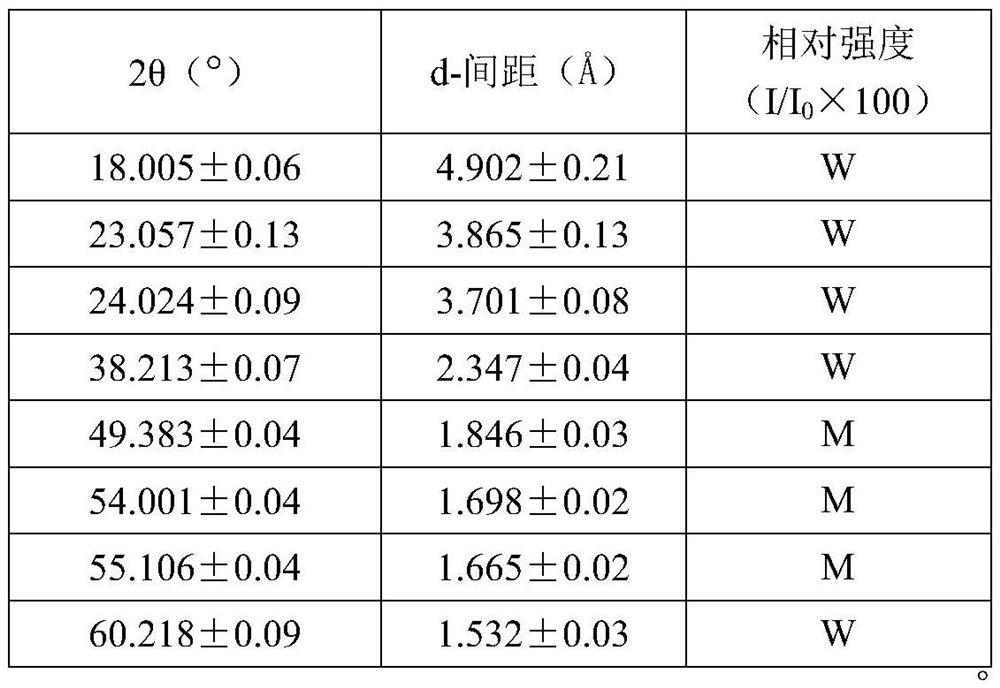
Image
Examples
Embodiment 1
[0044] 1. Preparation of catalyst
[0045] Ferric nitrate nonahydrate containing 1 mole of Fe (molecular formula is: Fe(NO 3 ) 3 9H 2 O), containing 50% manganese nitrate solution of 0.45 moles of Mn (molecular formula is: Mn(NO 3 ) 2 ), gallium nitrate pentahydrate containing 0.2 moles of Ga (molecular formula: Ga(NO 3 ) 3 ·5H 2 O), potassium nitrate containing 0.05 mole K (molecular formula: KNO 3 ) were sequentially dissolved in deionized water, and after complete dissolution, the metal ion mixed solution I was obtained. Dissolve 2.2 moles of ammonium carbamate in deionized water to obtain precipitant solution II after complete dissolution. The metal ion mixed solution I and the precipitant solution II were fed in parallel to carry out co-precipitation reaction, and aged at 20° C. for 36 hours to obtain the mixed slurry III. Send the mixed slurry III into a spray dryer, spray dry into microspherical fine particles, and then calcine at a temperature of 650°C for 8 h...
Embodiment 2
[0056] 1. Preparation of catalyst
[0057] Ferric nitrate nonahydrate containing 1 mole of Fe (molecular formula is: Fe(NO 3 ) 3 9H 2 O), containing 50% manganese nitrate solution of 0.30 moles of Mn (molecular formula is: Mn(NO 3 ) 2 ), gallium nitrate pentahydrate containing 0.10 moles of Ga (molecular formula: Ga(NO 3 ) 3 ·5H 2 O), potassium nitrate containing 0.10 mole K (molecular formula: KNO 3 ) were sequentially dissolved in deionized water, and after complete dissolution, the metal ion mixed solution I was obtained. Dissolve 2.2 moles of ammonium formate in deionized water to obtain precipitant solution II after complete dissolution. The metal ion mixed solution I and the precipitant solution II were fed in parallel to carry out co-precipitation reaction, and aged at 20° C. for 36 hours to obtain the mixed slurry III. Send the mixed slurry III into a spray dryer, spray dry into microspherical fine particles, and then calcine at a temperature of 650°C for 8 ho...
Embodiment 3
[0064] 1. Preparation of catalyst
[0065] Ferric nitrate nonahydrate containing 1 mole of Fe (molecular formula is: Fe(NO 3 ) 3 9H 2 O), containing 50% manganese nitrate solution of 0.60 moles of Mn (molecular formula is: Mn(NO 3 ) 2 ), zinc nitrate hexahydrate containing 0.01 mole of Zn (molecular formula: Zn(NO 3 ) 2 ·6H 2 O), lithium nitrate containing 0.15 moles of Li (molecular formula: LiNO 3 ) were sequentially dissolved in deionized water, and after complete dissolution, the metal ion mixed solution I was obtained. Dissolve 3.52 moles of ammonium carbamate in deionized water to obtain precipitant solution II after complete dissolution. The metal ion mixed solution I and the precipitant solution II are fed in parallel to carry out co-precipitation reaction, and aged at 30° C. for 1 hour to obtain the mixed slurry III. The mixed slurry III is sent to a spray dryer, spray-dried into microspherical fine particles, and then calcined at a temperature of 500°C for a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com