Substituted thiophenecarboxamides and analogues as antibacterials agents
A compound, methylpropyl technology, applied in the direction of compounds, biocides, disinfectants, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
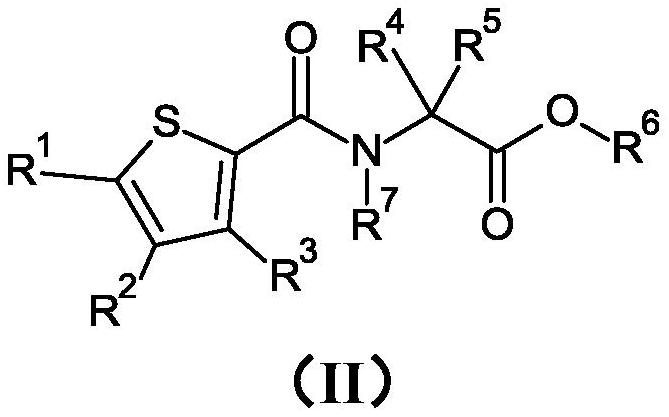
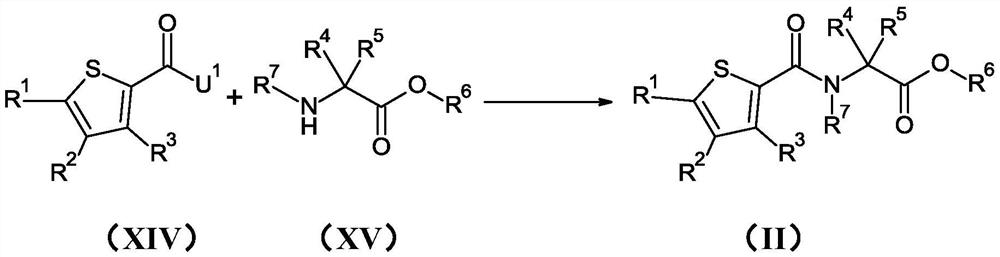
[0270] Preparation Example 1 : Preparation of N-[(3,4,5-trichloro-2-thienyl)carbonyl]glycine methyl ester (compound II.01)
[0271] To a solution of 250 mg (1.08 mmol) of 3,4,5-trichlorothiophene-2-carboxylic acid and 210 mg (1.67 mmol) of ethyl glycine hydrochloride (1:1) dissolved in 4.2 mL of tetrahydrofuran was added 0.23 mL (1.62 mmol) of triethylamine followed by the addition of 1.0 mL (1.68 mmol) of a 50% (w / w) solution of propanephosphonic anhydride in ethyl acetate. The reaction mixture was stirred at room temperature for 20 hours. The reaction mixture was quenched with water and extracted with ethyl acetate. The combined organic layers were dried over magnesium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (gradient n-heptane / ethyl acetate) to afford 178 mg (93% purity, 51% yield) of N-[(3,4,5-trichloro-2 -thienyl)carbonyl]glycine methyl ester. LogP = 2.8. (M+H)=302.
preparation Embodiment 2
[0272] Preparation Example 2 : Preparation of N-[(3,4,5-trichloro-2-thienyl)carbonyl]glycine (compound II.05)
[0273] To a solution of 178 mg (0.59 mmol) of N-[(3,4,5-trichloro-2-thienyl)carbonyl]glycine methyl ester dissolved in 3 mL of tetrahydrofuran, 1.3 mL of 1 M aqueous lithium hydroxide was added dropwise (1.3 mmol). The reaction was stirred at room temperature for 4 hours. The reaction mixture was diluted with ethyl acetate, water and saturated aqueous sodium bicarbonate solution. The organic layer was washed twice with saturated aqueous sodium bicarbonate solution. The combined aqueous layers were carefully acidified with 37% (w / w) aqueous hydrochloric acid at 0°C and extracted with ethyl acetate. The combined organic layers were dried over magnesium sulfate, filtered and concentrated under reduced pressure to afford 159 mg (98% purity, 92% yield) of N-[(3,4,5-trichloro-2- Thienyl)carbonyl]glycine. LogP = 2.14. (M-H)=286.
preparation Embodiment 3
[0274] Preparation Example 3 : Preparation of ethyl 1-{[(3,4,5-trichloro-2-thienyl)carbonyl]amino}cyclopropanecarboxylate (compound II.02)
[0275] step 1 : Preparation of ethyl 1-{[(3-amino-4,5-dichloro-2-thienyl)carbonyl]amino}cyclopropanecarboxylate (compound XVIIc.01)
[0276] To 150mg (0.60mmol) of 3-amino-4,5-dichlorothiophene-2-formic acid hydrochloride (1:1) (compound XIXc.01) and 255mg (1.50mmol) of 1-aminocyclopropane formic acid To a solution of ethyl ester hydrochloride (1:1) dissolved in 4.0 mL of dichloromethane was added 0.45 mL (2.59 mmol) of N,N-diisopropylethylamine, followed by 255 mg (1.50 mmol) of 2 - A solution of chloro-1,3-dimethylimidazolium chloride in 2.0 mL of dichloromethane. The reaction mixture was stirred at room temperature for 16 hours. The reaction mixture was quenched with water and extracted with dichloromethane. The combined organic layers were dried over magnesium sulfate, filtered and concentrated under reduced pressure. The resi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com