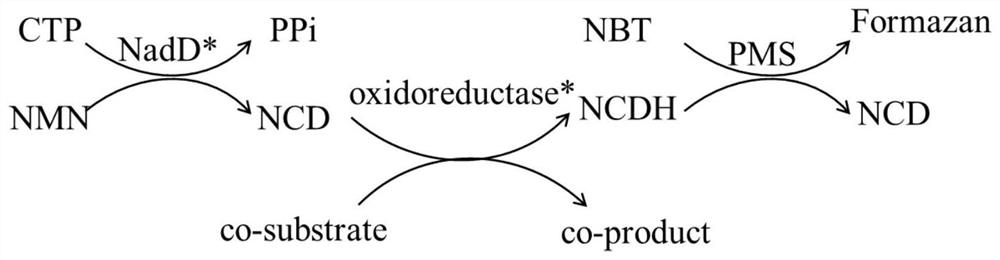
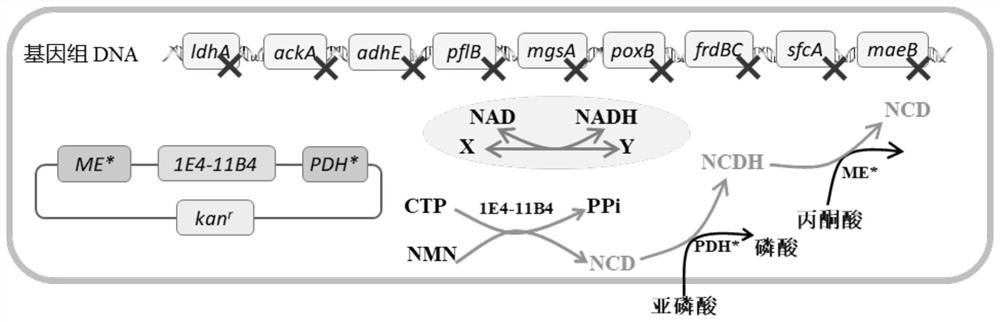
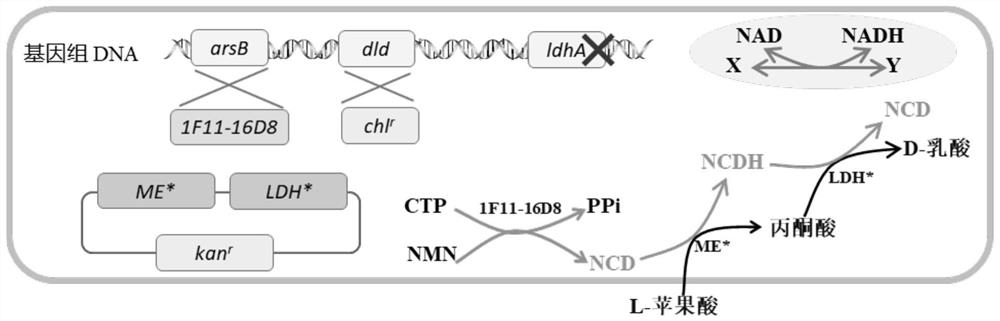
Method for enzymatic synthesis of nicotinamide cytosine dinucleotide and application thereof
A technology of nicotinamide cytosine dinucleotide and cytosine nucleoside triphosphate, which is applied in the directions of transferase, fermentation, etc., can solve the problems of difficult entry of NCD into cells, difficulty in separation and purification, and complicated steps, and achieve easy separation and reaction. The effect of high efficiency and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 122D8
[0078] Example 1 22D8 Catalyzed Synthesis of NCD
[0079] 1. Construction of mutant library
[0080] The mutation library was constructed by RF cloning (F. van den Ent, et al. Journal of Biochemical and Biophysical Methods. 2006, 67, 67). In the first step, the template is the wild-type NadD expression vector pUC-NadD, and the primers replace the codon of the 22nd proline with the degenerate base NNK (N=A,C,G,T; K=G,T) , carry out PCR reaction and recover the PCR product; the second step, the template is the same as the first step, and the primer is the PCR product recovered in the first step, and the obtained product is digested with DpnI restriction enzyme, and transferred into DH10B competent cells to obtain The transformant is a single point saturation mutation library that can express the mutation of proline at position P22 to any amino acid. The expression bacteria of each mutant are named DH10B (pUC-NadD-xxx), and the expressed protein product is named xxx. Among them...
Embodiment 21E
[0091] Example 2 Catalytic synthesis of NCD by 1E4
[0092] 1. Construction of mutant library
[0093] The mutation library was constructed by RF cloning (F. van den Ent, et al. Journal of Biochemical and Biophysical Methods. 2006, 67, 67). In the first step, the template is the mutant protein expression vector pUC-NadD-22D8 in Example 1, and the primers are the corresponding codons of cysteine at position 132 and tryptophan at position 175 are replaced with degenerate base groups CKC and TWT , SAA (equal molar ratio, K=G, T; W=A, T; S=C, G) primer pair, PCR reaction and recovery product; second step, template is the same as the first step, and primer is the first step The PCR product recovered in the medium was digested with DpnI restriction enzyme, and transferred to DH10B competent cells, and the obtained transformant was a mutation library capable of expressing NadD mutants, and each mutant expression strain was named as DH10B ( pUC-NadD-xxx), the expressed protein pro...
Embodiment 3
[0104] Embodiment 3 combinatorial mutant 1E4-11B4 catalyzes the synthesis of NCD
[0105] 1. Construction of 1E4-11B4 combined mutant expression vector
[0106] Combination mutant expression vectors were constructed using the method of RF cloning (F. van den Ent, et al. Journal of Biochemical and Biophysical Methods. 2006, 67, 67). In the first step, the template is the mutant protein expression vector pUC-NadD-11B4, the upstream primer is NadD-33-44-s (ggtctgacgcgggtcacaatcatccctaataatgttcctcc), the downstream primer is NadD-119-131-a (gacgatcaaatgtgcattgtcgagtatcgtttcgtattc), and the PCR reaction is carried out and the product is recovered In the second step, the template is the mutant protein expression vector pUC-NadD-1E4, and the primers are the PCR product recovered in the first step, and the product obtained is digested with DpnI restriction enzyme, and transferred into DH10B competent cells, and the obtained transformation The child is the expressable combination muta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com