Amlodipine dry suspension and preparation method thereof
A technology for dry suspensions and amlodipine besylate, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc. Low solubility and other issues, to achieve good market prospects, good stability, easy to carry the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
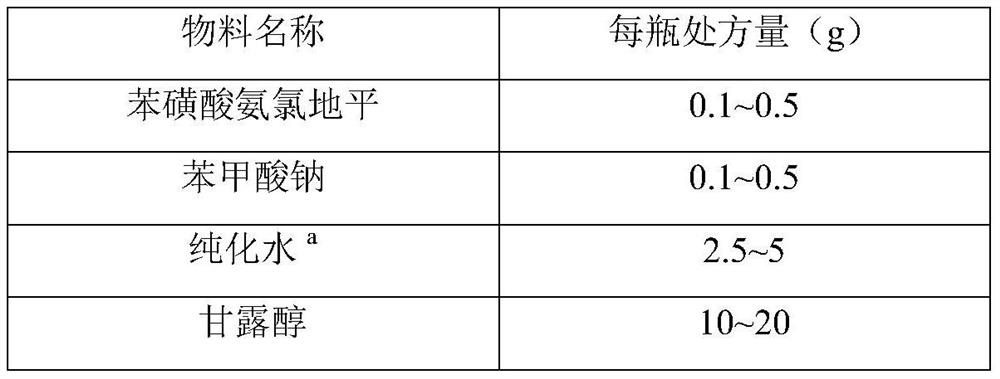
[0033] prescription:
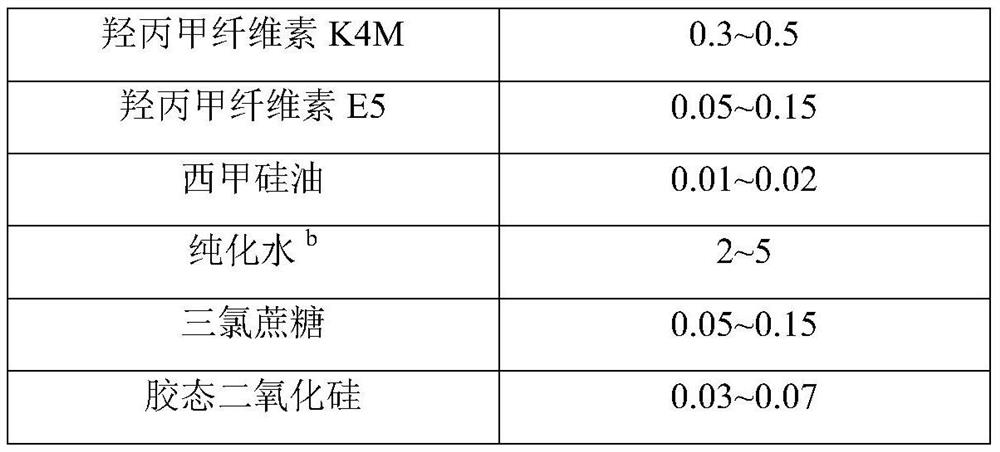
[0034] Material name Prescription amount Amlodipine 13.9g sodium benzoate 13.9g purified water a
1155.0g Mannitol 621.1g Hypromellose K4M 13.0g purified water b
200.0g
[0035] The preparation process is as follows:
[0036] a) in purified water a Amlodipine besylate in the prescription amount was added to it, prepared by a high-speed homogeneous process, with a speed of 5000 RPM, prepared for 2 minutes to make the dispersion uniform, and to obtain mixture 1;
[0037]b) Mixture 1 was prepared by adding the prescribed amount of sodium benzoate, prepared by a high-speed homogenization process at a speed of 10,000 RPM, prepared for 10 minutes, and prepared into Mixture 2 for later use;
[0038] c) Add mannitol and hypromellose K4M into the wet mixing granulator, and add purified water b Granulate, dry and granulate to obtain blank granules; add the blank granules to a fluidized bed granulation dr...
Embodiment 2
[0042] prescription:
[0043] Material name Prescription amount Amlodipine 13.9g sodium benzoate 28g purified water a
250.2g ethanol 27.8g Erythritol 677.1g Hypromellose K1500 31.9g Simethicone 1.0g
[0044] The preparation process is as follows:
[0045] a) in purified water a Add the prescribed amount of amlodipine besylate to the mixed solvent of ethanol, prepare by high-speed homogenization process, the speed is 8000RPM, prepare for 2 minutes to make the dispersion uniform, and obtain mixture 1;
[0046] b) Mixture 1 was prepared by adding the prescribed amount of sodium benzoate, prepared by a high-speed homogenization process at a speed of 8000 RPM, and prepared for 10 minutes to prepare mixture 2 for later use;
[0047] c) adding erythritol, hypromellose K1500 and simethicone into the fluidized bed granulation dryer, spraying into the mixture 2 prepared in step b, and performing one-step granulation;
[004...
Embodiment 3
[0051] prescription:
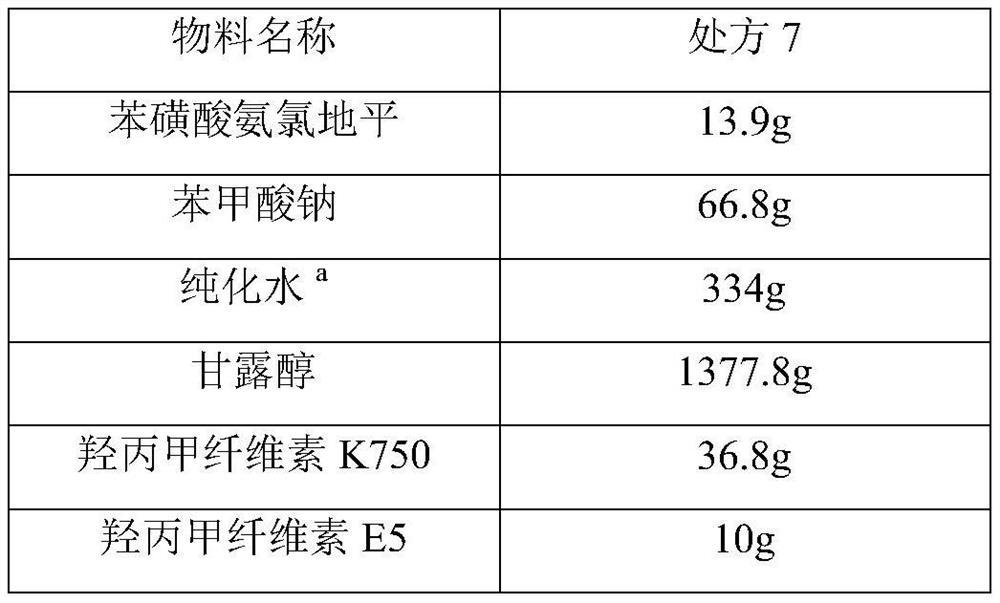
[0052] Material name Prescription 7 Amlodipine 13.9g sodium benzoate 66.8g purified water a
334g Maltitol 1488.0g Erythritol 600.1 Hypromellose K750 33.2g Simethicone 1.3g aspartame 15g talcum powder 4.2g
[0053] The preparation process is as follows:
[0054] a) in purified water a Amlodipine besylate in the prescribed amount was added to it, prepared by a high-speed homogeneous process, with a speed of 8000 RPM, prepared for 2 minutes to make the dispersion uniform, and to obtain mixture 1;
[0055] b) Mixture 1 was prepared by adding the prescribed amount of sodium benzoate, prepared by a high-speed homogenization process at a speed of 8000 RPM, and prepared for 10 minutes to prepare mixture 2 for later use;
[0056] c) Add maltitol, erythritol, hypromellose K750 and simethicone into the fluidized bed granulation dryer, spray the mixture 2 prepared in step b, and perform one-s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com