High-stability water-borne epoxy resin and preparation method thereof
A water-based epoxy resin with high stability technology, applied in the field of coating materials, can solve the problems of high bond strength, high hardness, poor heat resistance and impact damage resistance of epoxy resin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
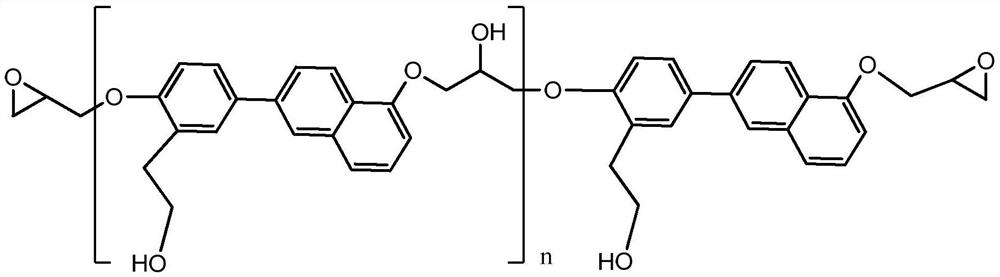
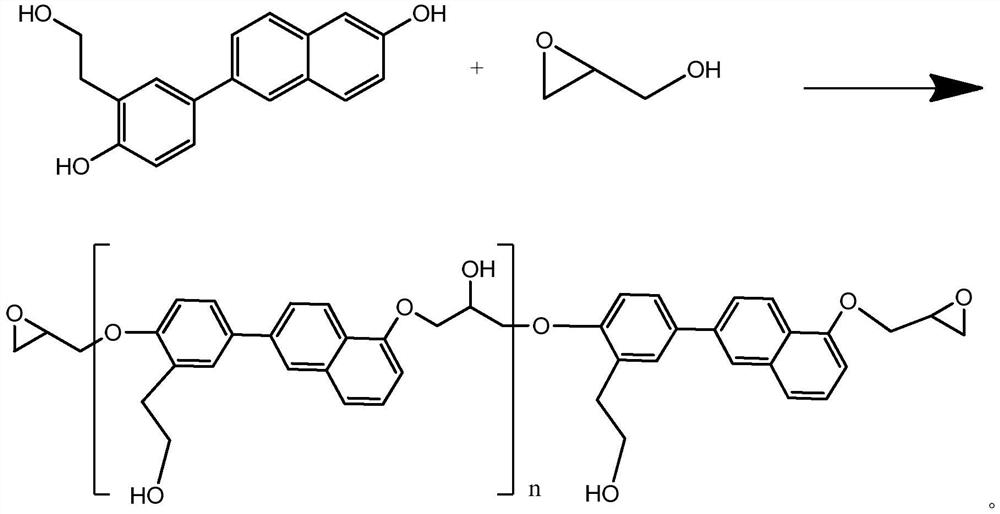
[0022] Dissolve the compound represented by formula II in toluene, add aqueous sodium hydroxide solution, heat while stirring, stir at a constant temperature when the temperature rises to 65°C, and add glycidol dropwise, and dropwise complete within 2 hours; raise the temperature , kept at 80°C for 1.5h; add distilled water, stir, statically separate and remove the water layer, evaporate the toluene layer to dryness under reduced pressure to obtain the compound shown in formula I; the molar ratio of the compound shown in formula II to glycidyl alcohol is n: (2n+2 to 2n+4); the content of sodium hydroxide in the aqueous sodium hydroxide solution is 0.5n-1.5n moles; the volume ratio of distilled water and toluene is 3:1.
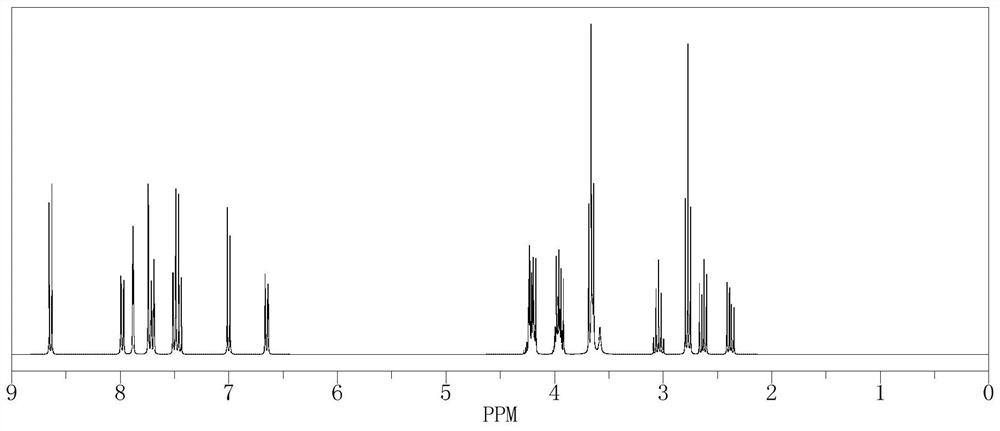
[0023] Obtain reaction product 569.7g, m / z 728.30, hydrogen spectrum sees figure 1 .
Embodiment 2
[0025] Dissolve the compound shown in formula II in toluene, add aqueous sodium hydroxide solution, heat while stirring, when the temperature rises to 60°C, stir at a constant temperature and add glycidol dropwise, and the dropwise addition is completed within 2 hours; raise the temperature , kept at 75°C for 2h; add distilled water, stir, statically separate and remove the water layer, evaporate the toluene layer under reduced pressure to obtain the compound shown in formula I; the molar ratio of the compound shown in formula II to glycidyl alcohol is n:( 2n+2 to 2n+4); the content of sodium hydroxide in the aqueous sodium hydroxide solution is 0.5n-1.5n moles; the volume ratio of distilled water and toluene is 4:1.
[0026] Obtain reaction product 469.4g, m / z 728.30, hydrogen spectrum see figure 1 .
Embodiment 3
[0028] Dissolve the compound shown in formula II in toluene, add aqueous sodium hydroxide solution, heat while stirring, when the temperature rises to 70°C, stir at a constant temperature and add glycidol dropwise, and the dropwise addition is completed within 2 hours; raise the temperature , kept at 85°C for 1h; add distilled water, stir, statically separate and remove the water layer, evaporate the toluene layer to dryness under reduced pressure to obtain the compound shown in formula I; the molar ratio of the compound shown in formula II to glycidyl alcohol is n:( 2n+2 to 2n+4); the content of sodium hydroxide in the aqueous sodium hydroxide solution is 0.5n-1.5n moles; the volume ratio of distilled water and toluene is 2:1.
[0029] Obtain reaction product 416.7g, m / z 728.30, hydrogen spectrum sees figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com