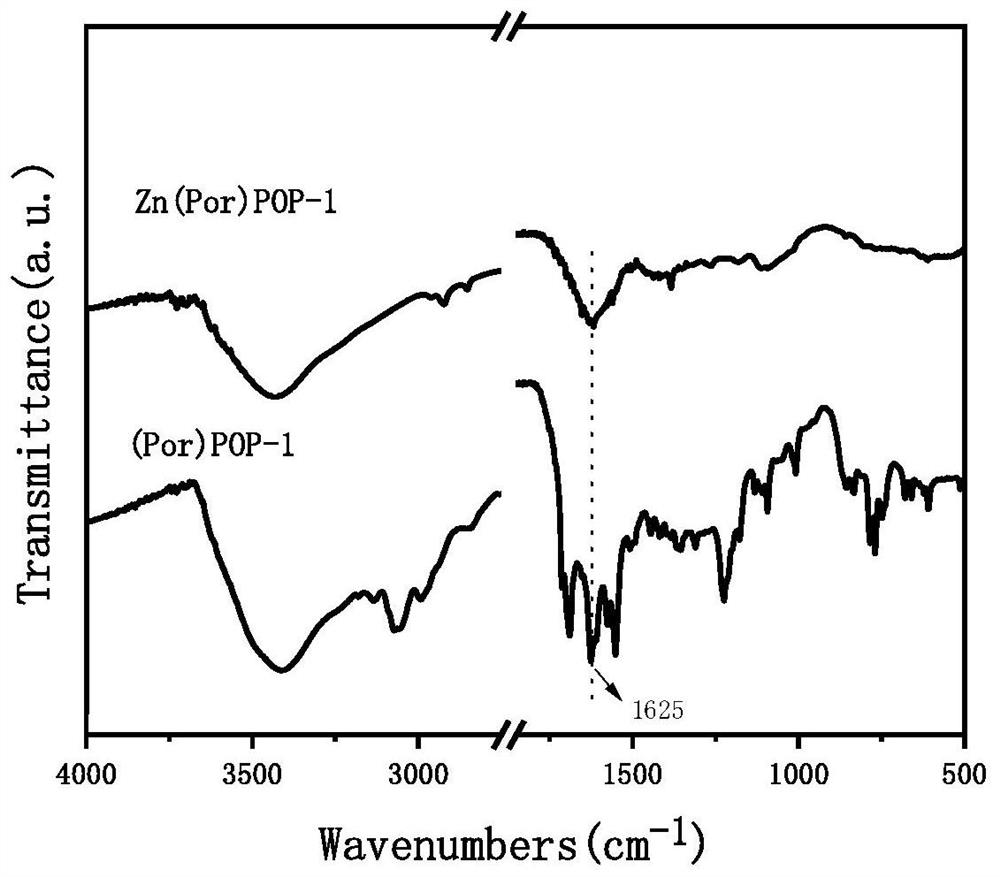
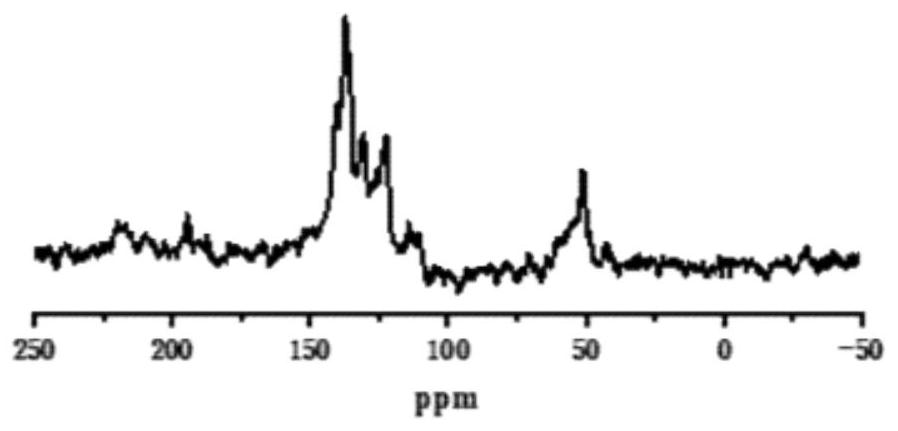
A kind of porphyrin-based porous organic polymer and its preparation method and the synthesis method of cyclic carbonate
A technology of cyclic carbonates and synthesis methods, applied in organic compound/hydride/coordination complex catalysts, organic chemistry, chemical instruments and methods, etc., can solve the problems of cumbersome conditions, increased production costs, and energy consumption. Achieve broad application prospects and good catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The chemical structure expression of the porphyrin-based porous organic polymer prepared in this example is: R-(R 1 ) m , where R 1 It has the structure shown in formula (I), m is 3, R is M is Zn and X is Br.
[0051] In this example, the above compounds are used as examples to prepare porphyrin-based porous organic polymers. The porphyrin-based porous organic polymers in other examples in this application can be prepared by selecting the corresponding raw materials and dosages by using the preparation method of this example.
[0052] 1. DMF ( 5 mL), the mixture solution was stirred for 30 minutes, then heated to 80° C., and stirred under a nitrogen atmosphere for 24 hours. After cooling to room temperature, the mixture was filtered and washed with ethanol, then dried under vacuum to give the intermediate compound.
[0053] 2. Under nitrogen protection and at 120°C, slowly drop the propionic acid of pyrrole into the propionic acid solution of the above intermediat...
Embodiment 2
[0057] Porphyrin-based porous organic polymer catalyst: m is 3, R is M is Zn and X is Br.
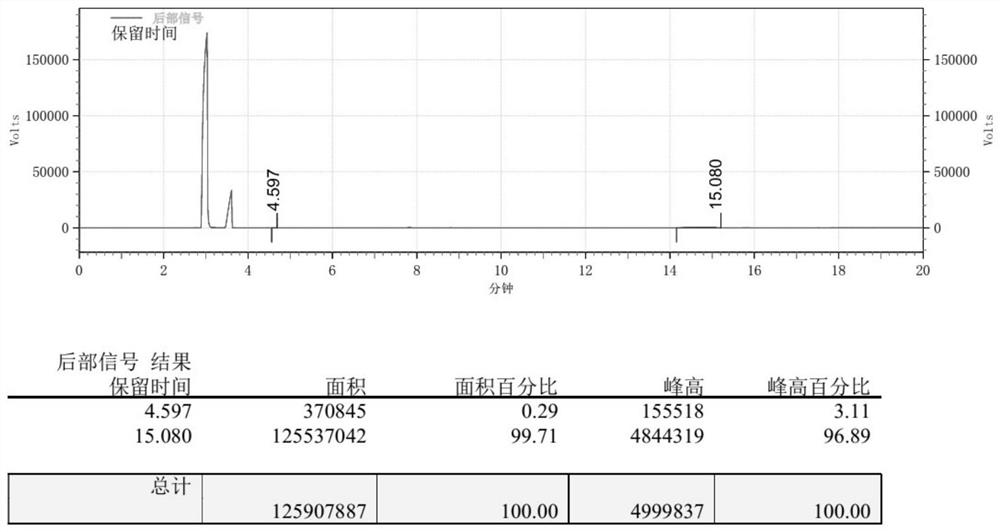
[0058] Into a 10mL stainless steel autoclave, add 7×10 -3 mmol of catalyst, 5mmol of epichlorohydrin, introduced carbon dioxide to make the initial pressure 1.0MPa, stirred for 5h under the condition of temperature of 80 ℃, quickly placed in cold water and cooled to room temperature, and then placed in ice water to continue cooling, After slowly releasing the remaining carbon dioxide, the catalyst was isolated by filtration. Get an appropriate amount of filtrate and carry out gas chromatographic analysis, the gas chromatogram of the product obtained in Example 2 of the application is as follows image 3 As shown, the yield of the obtained cyclic carbonate was 99%.
Embodiment 3
[0060] Porphyrin-based porous organic polymer catalyst: m is 3, R is M is Zn and X is Br.
[0061] Into a 10mL stainless steel autoclave, add 7×10 -3 mmol of the catalyst, 5mmol of epichlorohydrin, introduced carbon dioxide and kept the pressure at 0.1MPa, stirred for 16h at a temperature of 80°C, cooled in ice water, slowly released the remaining carbon dioxide, and separated the catalyst by filtration. An appropriate amount of the filtrate was taken and analyzed by gas chromatography, and the yield of the obtained cyclic carbonate was 92%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap