Medium-temperature bacterium for producing high-salinity tolerance ester hydrolase and application of medium-temperature bacterium
A hydrolytic enzyme and high-salinity technology, applied in the field of microorganisms, can solve the problems of restricting practical applications, difficulty in meeting practical application requirements, and inhibition, and achieve the effects of low cost, strong thermal stability, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Activity detection of ester hydrolase Ala2 produced by mesophilic bacteria SIOC 00011
[0047] The activity of the purified ester hydrolase was determined by the p-nitrophenol hexanoate method. Specific operation: 1 ml reaction system includes 1 mM p-nitrophenol hexanoate, 100 mM NaH 2 PO 4 -Na 2 HPO 4 Buffer solution (pH=8.0) and 0.0016 μg of pure enzyme protein, using a UV-visible spectrophotometer (Beckman DU800, USA) to continuously measure the absorbance value A at 45 °C 405 2 min, use the inactivated enzyme solution as a control for zero adjustment. One unit of enzyme activity is defined as the amount of enzyme required to catalyze the production of 1 µmol p-nitrophenol from p-nitrophenol esters per minute. The measured esterase activity was 14586 U / mg.
Embodiment 2
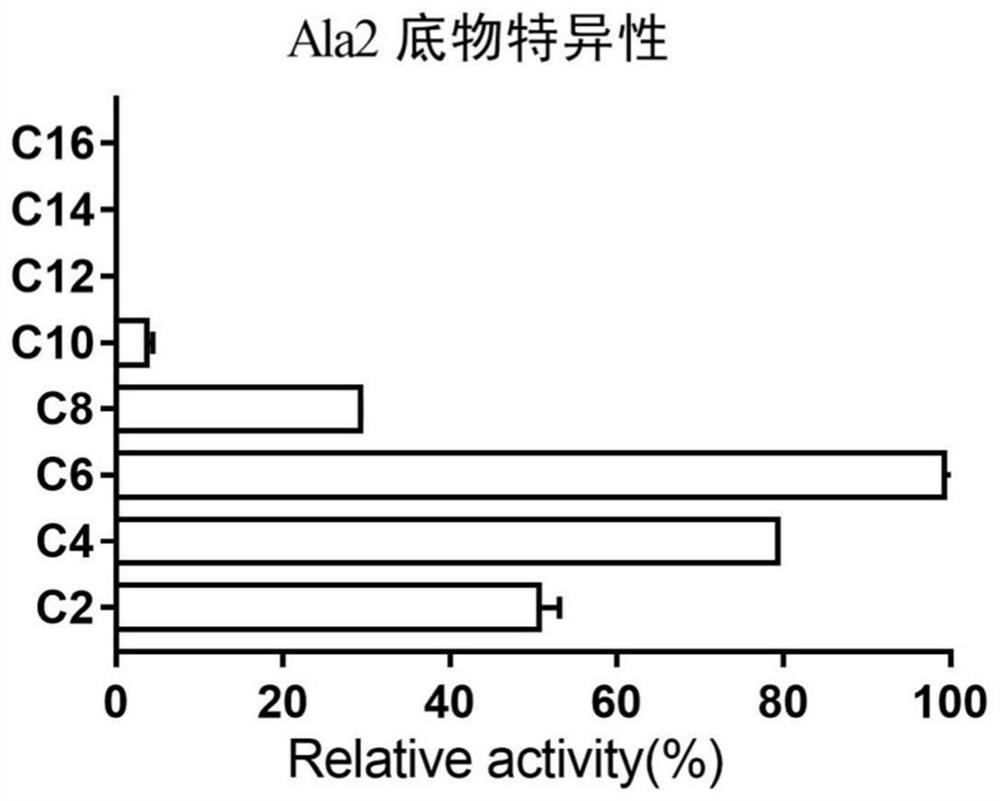
[0048] Embodiment 2: Ester hydrolase Ala2 substrate specificity analysis
[0049] The substrate specificity analysis of hydrolase Ala2 uses the system (1 ml): 100 mM NaH 2 PO 4 -Na 2 HPO 4 Buffer (pH=8.0), 1 mM substrate, add 0.0016 μg pure enzyme protein, and measure the absorbance value A continuously at 45°C 405 2 min. The substrates used in the determination are: p-nitrophenol acetate (C2), p-nitrophenol butyrate (C4), p-nitrophenol hexanoate (C6), p-nitrophenol octanoate (C8) , p-nitrophenol caprate (C10), p-nitrophenol dodecanoate (C12), p-nitrophenol myristate (C14), p-nitrophenol palmitate (C16). It has been determined that Ala2 has higher catalytic activity for p-nitrophenol esters with shorter acyl carbon chains (C2, C4, C6 and C8), and the highest catalytic activity is when the substrate is p-nitrophenol hexanoate (C6). ( figure 1 ). The results showed that ester hydrolase Ala2 had better catalytic activity on esters with shorter acyl carbon chains, and it...
Embodiment 3
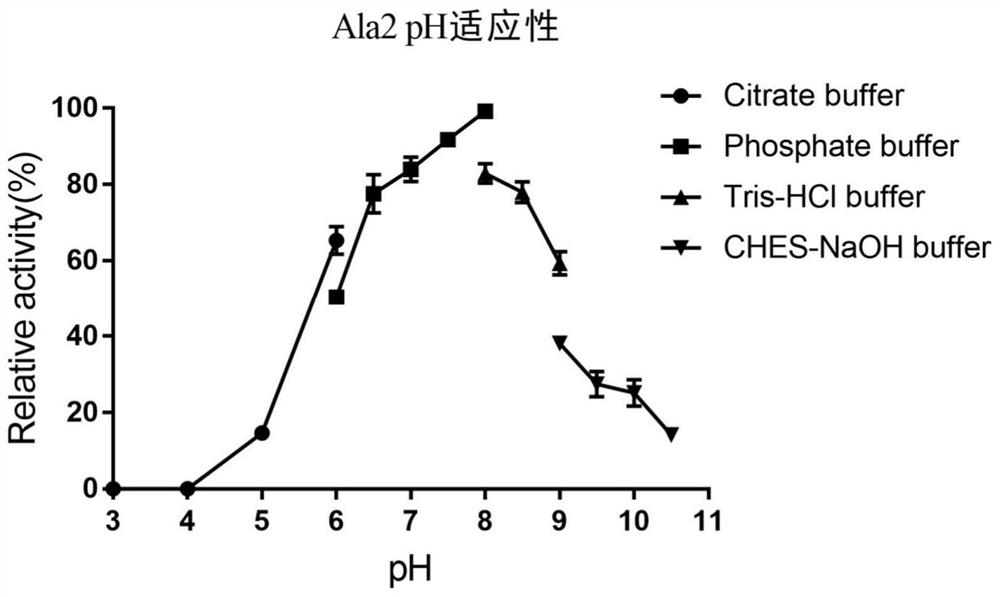
[0050] Embodiment 3: analysis of optimal reaction conditions of ester hydrolase Ala2
[0051] The optimal reaction pH of hydrolase Ala2 was determined in the range of 4.0 to 10.5. The specific operation is: add 1 mM p-nitrophenol hexanoate and 0.0016 μg pure enzyme protein to different pH buffers, and continuously measure the absorbance value A at 45°C 348 2 min. The buffer used for the determination is: 100 mM citric acid-sodium citrate buffer (pH 3.0~6.0), 100 mM potassium dihydrogen phosphate-sodium hydroxide buffer (pH 6.0~8.0), 100 mM Tris hydrochloric acid buffer ( pH 7.5~9.0) and 50 mM 2-cyclohexylaminoethanesulfonic acid-sodium hydroxide buffer (pH 9.0~10.5). The measurement results showed that the optimal reaction pH of Ala2 was 6.0, and it was active in the range of pH 5.0-10.5 ( figure 2 ).
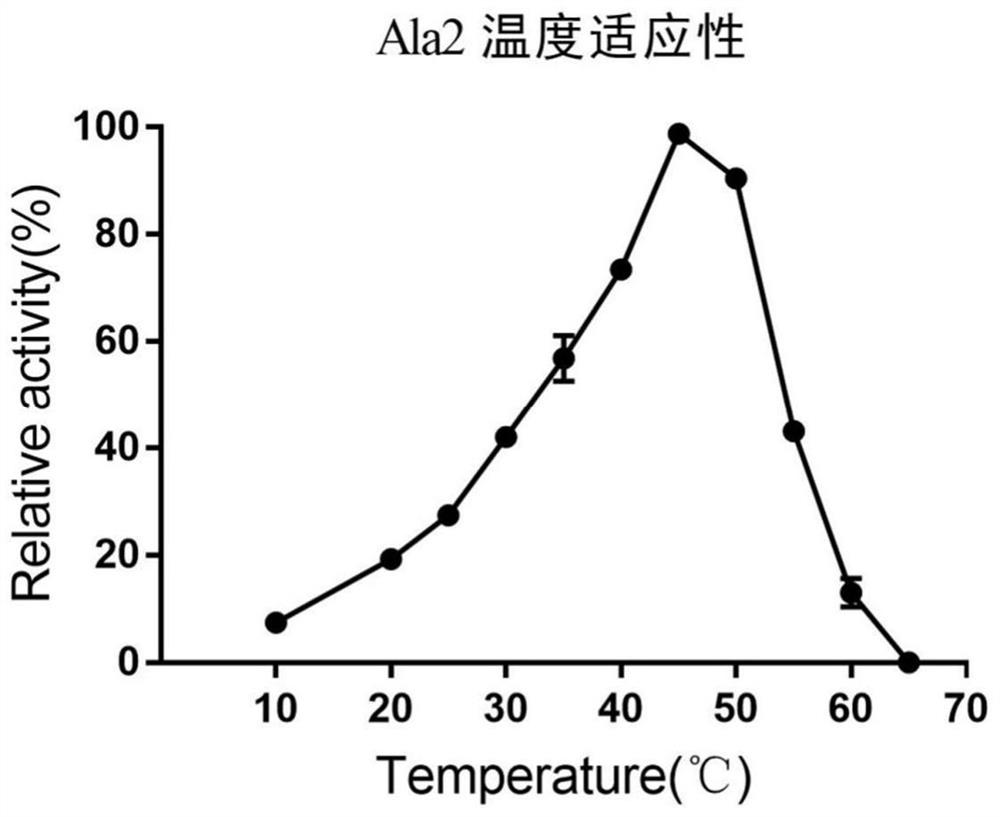
[0052] The optimal reaction temperature of hydrolase Ala2 was determined in the range of 25-55 degrees Celsius. The specific operation is: in 1 ml reaction system, add 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Absorbance | aaaaa | aaaaa |
| Absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com