Resveratrol lamellar liquid crystal and preparation method thereof
A technology of resveratrol and lamellar liquid crystals, which can be used in pharmaceutical formulations, medical preparations of non-active ingredients, drug delivery, etc. It can solve the problems of inability to stay in the cornea for a long time, reduce patient compliance, and increase adverse reactions. , to achieve the effects of inhibiting corneal neovascularization, promoting corneal epithelial repair, and enhancing drug permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] The present invention will be described in detail below by way of examples. The examples of said embodiments are intended to explain the invention and are not to be construed as limiting the invention. If no specific technique or condition is indicated in the examples, it shall be carried out according to the technique or condition described in the literature in this field or according to the product specification. The reagents or instruments used were not indicated by the manufacturer, and they were all commercially available conventional products.
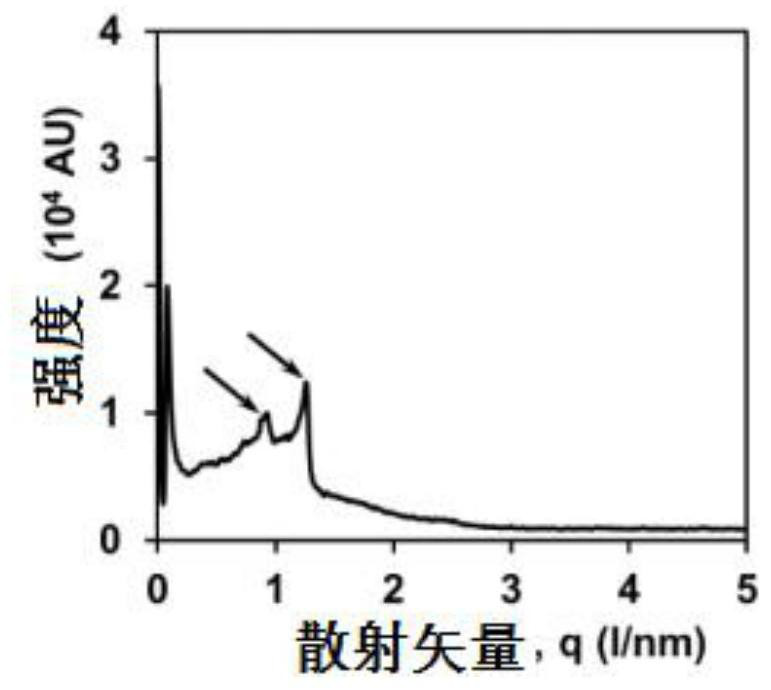
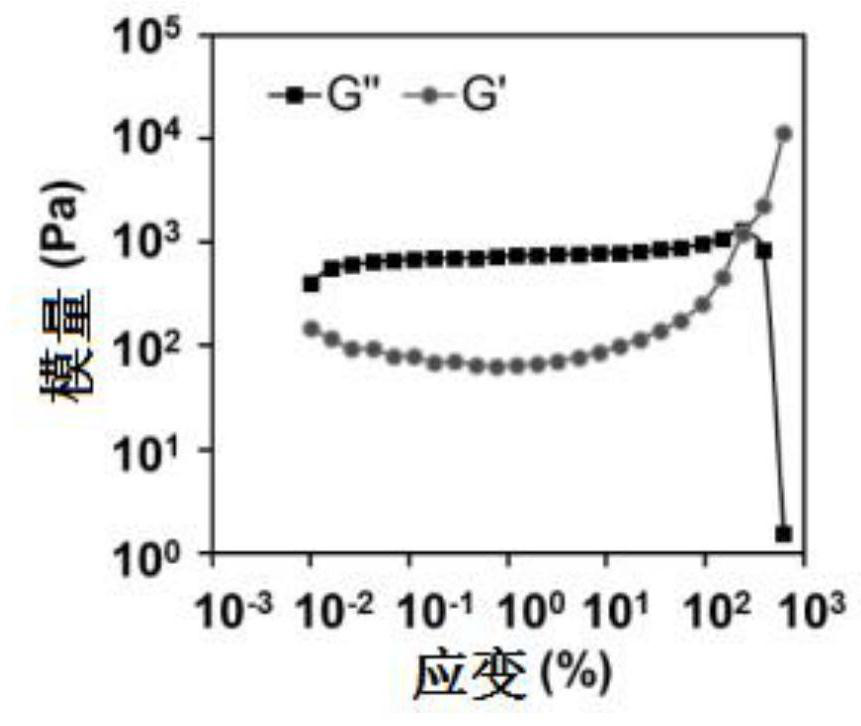
[0037] Preparation and Characterization of Resveratrol Lamellar Liquid Crystals (ROLG)
[0038] 1. Experimental materials
[0039] (1) Experimental reagents:
[0040]
[0041] (2) Experimental equipment:
[0042]
[0043] 2. Experimental method
[0044] (1) Preparation of Resveratrol Lamellar Liquid Crystals
[0045] Resveratrol (42.0 mg) was dissolved in ethanol (0.6 g) to obtain a resveratrol-ethanol solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com