Tetrazine compound as well as preparation method and application thereof
A compound and reaction solvent technology, applied in the field of tetrazine labeling, can solve problems such as poor selectivity, greater influence on the activity of biomolecules itself, and poor stability of coupling products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
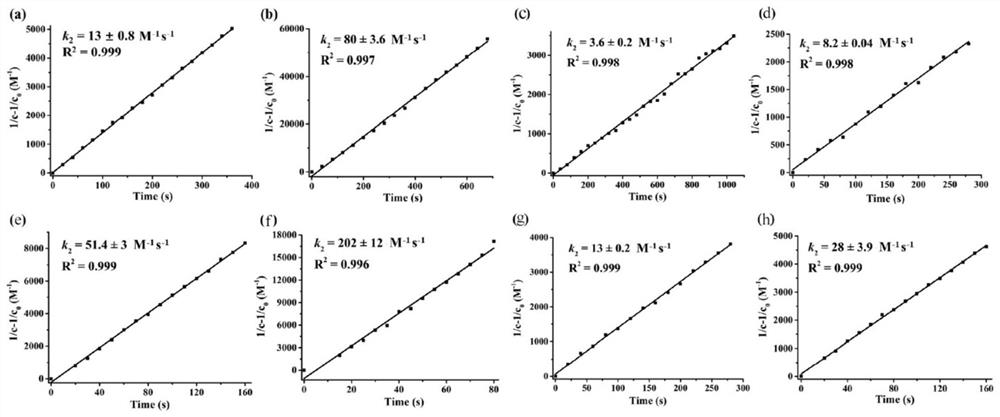
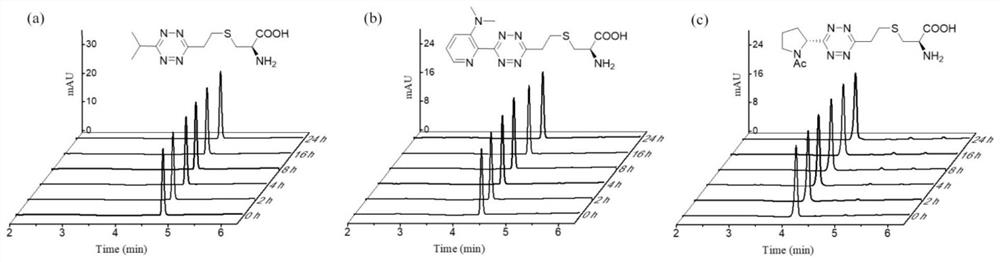
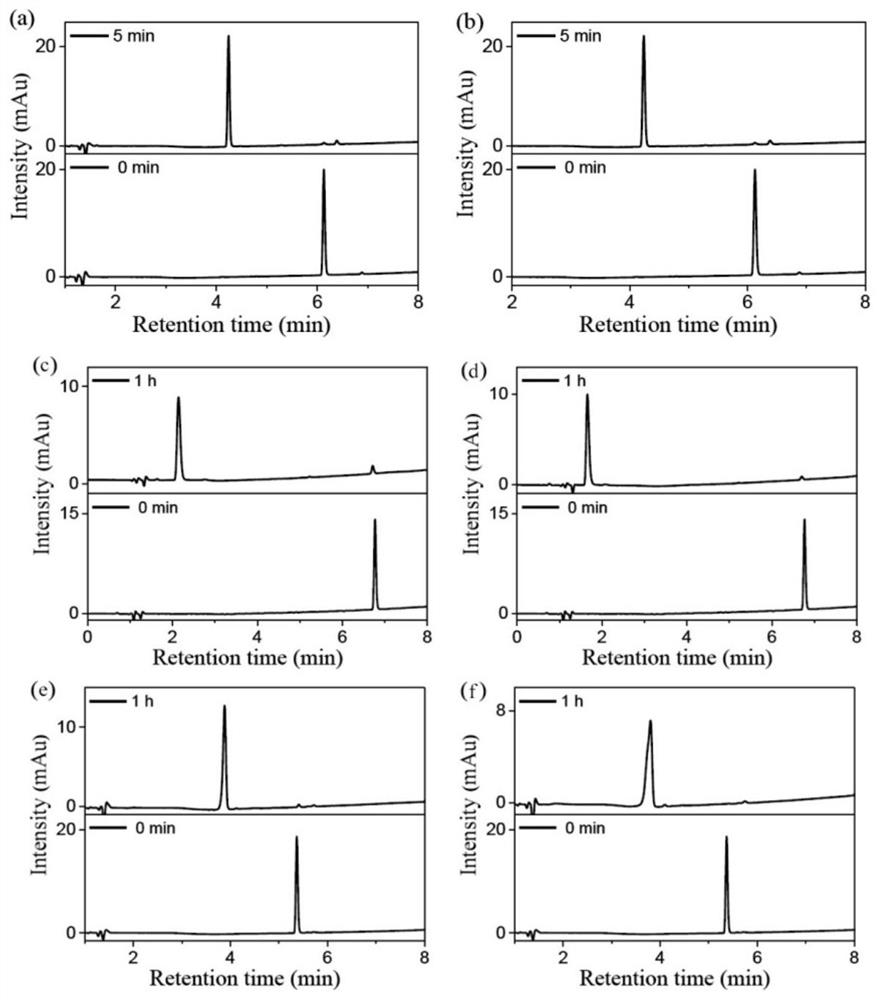
Image
Examples
Embodiment 1
[0089] Synthesis of 3-phenyl-6-vinyl-1,2,4,5-tetrazine:
[0090]
[0091] Benzonitrile (206 mg, 2 mmol), 3-hydroxypropionitrile (548 μL, 8 mmol), 3-mercaptopropionic acid (174 μL, 3 mmol), ethanol (0.6 mL) and hydrazine hydrate (1.2 mL, 24 mmol) were added to a A 10 mL reaction vessel with a magnetic stir bar. Under the protection of argon, the reaction solution was stirred overnight at 40°C. After that, the reaction solution was cooled to 0° C., and sodium nitrite (1.38 g, 20 mmol) dissolved in ice water (50 mL) was slowly added to the reaction solution. Then slowly add 1M HCl under an ice-water bath, the reaction solution turns bright red and gas is generated, then continue to add HCl until the generation of gas stops, the pH value is 1-6, preferably, the pH value is 1-4, more preferably 2 ~3. The resulting mixture was further stirred for about 5 minutes, extracted with dichloromethane (50 mL x 3), dried over anhydrous sodium sulfate, concentrated in vacuo, and purifie...
Embodiment 2
[0100]Synthesis of 3-(2-methoxyethyl)-6-vinyl-1,2,4,5-tetrazine:
[0101]
[0102] 3-Methoxypropionitrile (190 mg, 2 mmol), 3-hydroxypropionitrile (548 μL, 8 mmol), 3-mercaptopropionic acid (174 μL, 3 mmol), ethanol (0.6 mL) and hydrazine hydrate (1.2 mL, 24 mmol) Add to a 10 mL reaction vessel equipped with a magnetic stir bar. Under the protection of argon, the reaction solution was stirred at room temperature for 16 hours. After that, the reaction solution was cooled to 0° C., and sodium nitrite (1.38 g, 20 mmol) dissolved in ice water (50 mL) was slowly added to the reaction solution. Then slowly add 1M HCl under an ice-water bath, the reaction solution turns bright red and gas is generated, then continue to add HCl until the generation of gas stops, the pH value is 1-6, preferably, the pH value is 1-4, more preferably 2 ~3. The mixture was further stirred for about 5 minutes, extracted with dichloromethane (50mL x6), dried over anhydrous sodium sulfate, concentrated...
Embodiment 3
[0111] Synthesis of 3-isopropyl-6-vinyl-1,2,4,5-tetrazine:
[0112]
[0113] 2-Methylalanine hydrochloride (292 mg, 2.6 mmol), 3-hydroxypropionitrile (712 μL, 10.4 mmol), 3-mercaptopropionic acid (226 μL, 2.6 mmol), ethanol (0.7 mL) and hydrazine hydrate (1.5 mL, 30 mmol) was added to a 10 mL reaction vessel equipped with a magnetic stir bar. Under the protection of argon, the reaction solution was stirred overnight at room temperature. After that, the reaction solution was cooled to 0° C., and sodium nitrite (1.8 g, 26 mmol) dissolved in ice water (50 mL) was slowly added to the reaction solution. Then slowly add 1M HCl under an ice-water bath, the reaction solution turns bright red and gas is generated, then continue to add HCl until the gas generation stops, the pH value is 1-6, preferably, the pH value is 1-4, more preferably 2~3. The mixture was further stirred for about 5 minutes, extracted with dichloromethane (50 mL x 5), dried over anhydrous sodium sulfate, conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com