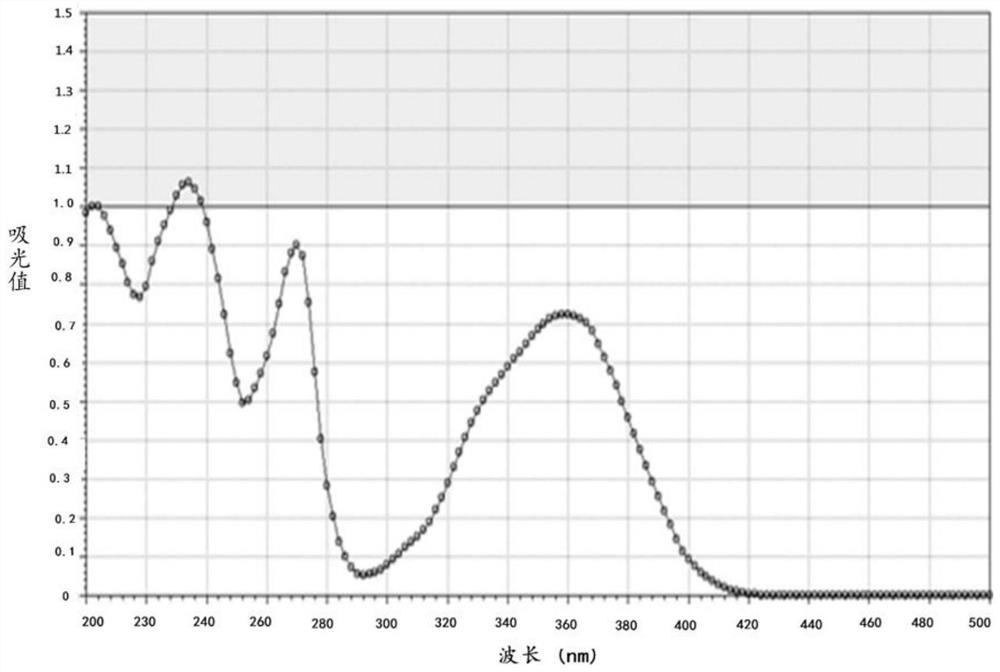
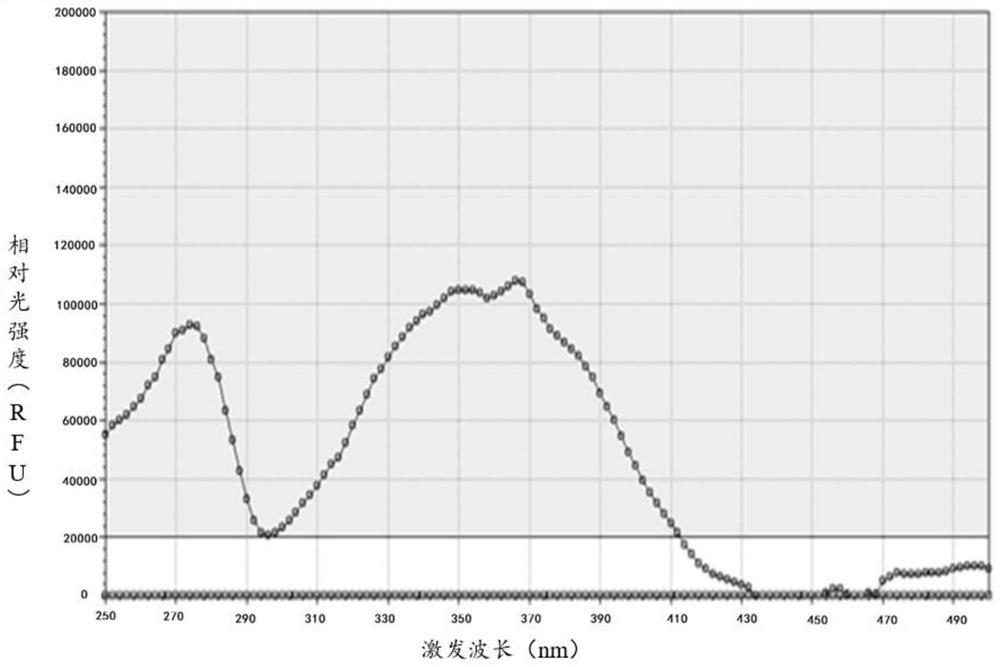
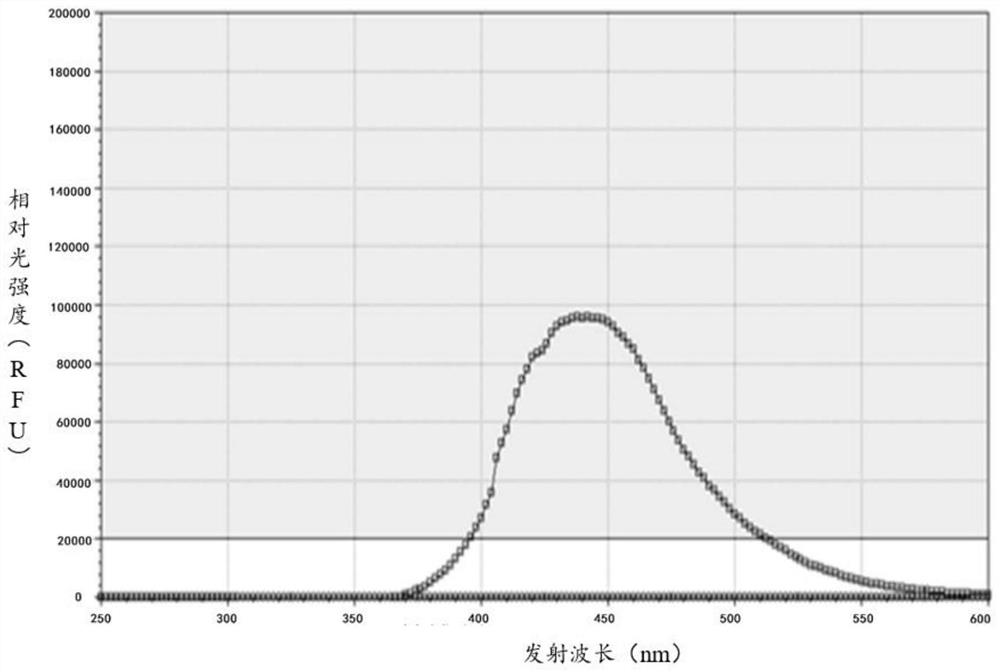
Quinoline derivative as well as preparation method and application thereof
A derivative, quinoline technology, applied in the field of quinoline derivatives and their preparation, can solve the problems of limitations, lack of universality, quinoline derivative fluorescein cannot be used to label small molecular compounds, etc., and meet the reaction conditions Mild, broadened selection range, and stable fluorescence properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] This embodiment provides a quinoline derivative of the present invention and a preparation method thereof.
[0073] Concrete preparation steps are as follows:
[0074] (1) 4-benzoyl butyric acid is subjected to amidation reaction to obtain compound I:
[0075] Dissolve 119g (100mmol) of 4-benzoylbutyric acid in 500mL of dichloromethane (DCM), then add 8.97g (110mmol) of dimethylamine hydrochloride, 50mL of N,N-diisopropylethylamine (DIPEA ), 41.80g (110mmol) tetramethylurea hexafluorophosphate (HATU), stirred at room temperature 25°C for 16h, after the reaction, washed with 500mL water each time, repeated twice, then dried with anhydrous sodium sulfate, centrifuged The precipitated substance was taken and separated by thin-layer chromatography (the developing solvent was dichloromethane / methanol (v / v, 50:1)) to obtain 14.25 g of compound I with a yield of 65%.
[0076] (2) subjecting the compound I to an oximation reaction to obtain compound II;
[0077] 14.25g (65mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com