A kind of preparation method of tedizolid phosphate freeze-dried preparation for injection
A technology of tedizolid phosphate and freeze-dried preparations, which is applied in the field of preparation of tedizolid phosphate freeze-dried preparations for injection, which can solve the problems of time-consuming, complicated freeze-drying process, and influence on the popularization and use of tedizolid phosphate freeze-dried preparations, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
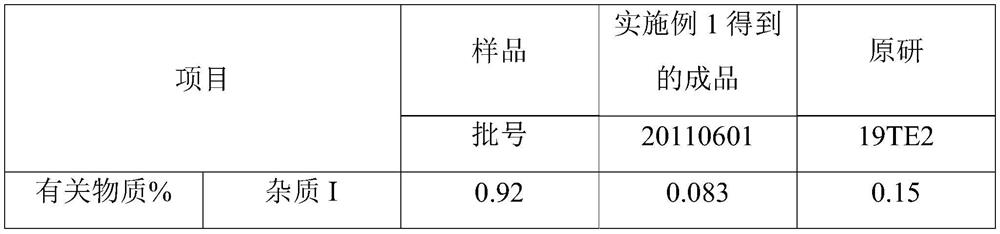
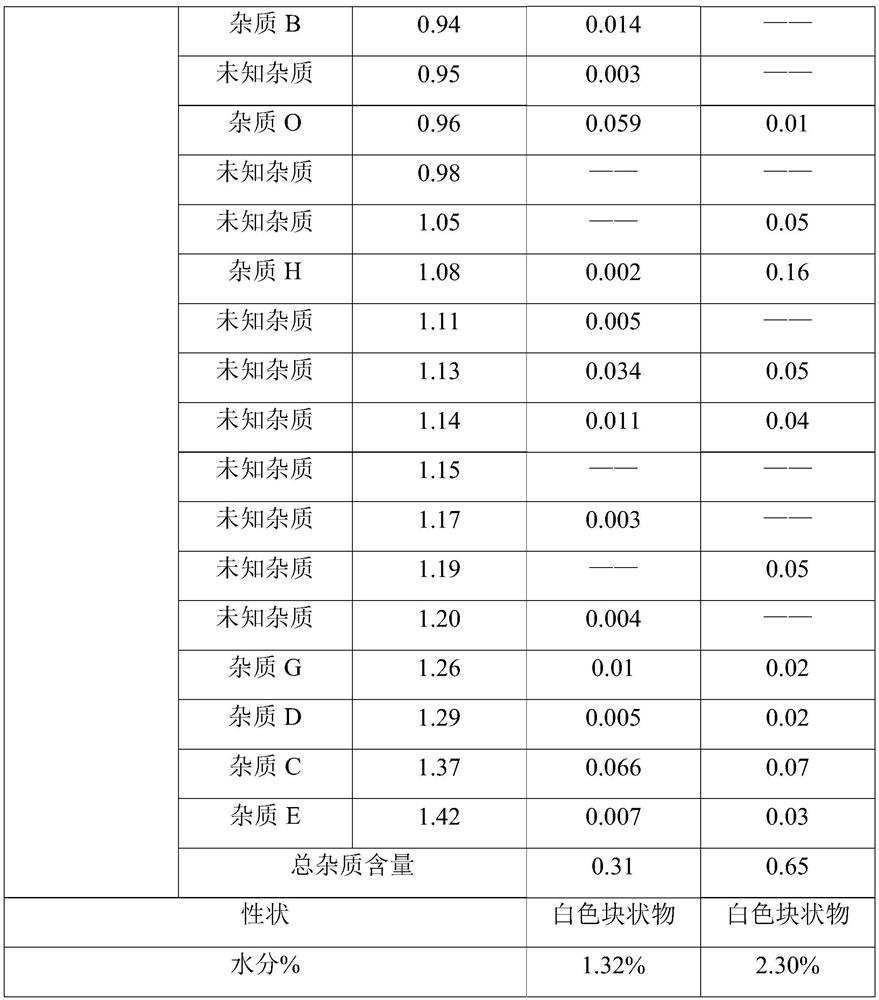
[0023] Take 500mL of fresh water for injection in a beaker, drop 126g of tedizolid phosphate, add dropwise 1mol / L sodium hydroxide solution until tedizolid phosphate is completely dissolved, add 63g of mannitol, measure pH, use 1mol / L Sodium hydroxide was used to adjust the pH to 7.7, water for injection was added to make the volume to 1260 mL, and the mixture was evenly mixed to obtain tedizolid phosphate medicinal solution. Fill according to the volume of 2.5ml / cartridge, fill 500 bottles, half stopper and move into the freeze dryer.
[0024] The freeze-drying steps in the freeze-dryer after the tedizolid phosphate medicinal solution is subpackaged are as follows:
[0025] Cool the tedizolid phosphate solution to -9°C at a rate of 1°C / min and keep it for 30 minutes, continue to cool at a rate of 1°C / min to -45°C and keep it for 40 minutes; then raise the temperature to -12°C at a rate of 1°C / min ℃ for 60 min; then cooled to -45 °C at a rate of 1 °C / min and kept for 90 min t...
Embodiment 2
[0027] Take 500mL of fresh water for injection in a beaker, drop 125g of tedizolid phosphate, add dropwise 0.5mol / L sodium hydroxide solution until tedizolid phosphate is completely dissolved, add 57g of mannitol, measure pH, use 0.5mol / L 1 L of sodium hydroxide to adjust the pH to 7.8, add water for injection to make the volume to 1000 mL, and mix evenly to obtain tedizolid phosphate medicinal solution. Fill according to the volume of 2ml / cartridge, fill 500 bottles, half stopper and move into the freeze dryer.
[0028] The freeze-drying steps in the freeze-dryer after the tedizolid phosphate medicinal solution is subpackaged are as follows:
[0029] Cool the tedizolid phosphate liquid at a speed of 0.8°C / min to -8°C and keep it for 25 minutes, continue cooling at a speed of 0.8°C / min to -40°C and keep it for 35 minutes; ℃ for 50 minutes; then cooled to -40 ℃ at a rate of 0.8 ℃ / min and kept for 80 minutes to obtain a lyophilized product; at this time, the chamber in the lyop...
Embodiment 3
[0031] Take 500mL of fresh water for injection in a beaker, put 200g of tedizolid phosphate into it, add dropwise 2mol / L sodium hydroxide solution until tedizolid phosphate is completely dissolved, add 110g of mannitol, measure the pH, use 1mol / L Adjust the pH to 7.7 with hydrochloric acid, add water for injection to make up to 2400mL, and mix well to obtain tedizolid phosphate liquid. Fill according to the volume of 3ml / cartridge, fill 800 bottles, half stopper and move into the freeze dryer.
[0032] The freeze-drying steps in the freeze-dryer after the tedizolid phosphate medicinal solution is subpackaged are as follows:
[0033] Cool the tedizolid phosphate liquid at a rate of 1.2 °C / min to -10 °C for 35 min, continue to cool at a rate of 1.2 °C / min to -50 °C and maintain for 45 min; then heat up to -10 °C at a rate of 1.2 °C / min ℃ for 70 min; then cooled to -50 °C at a rate of 1.2 °C / min and kept for 100 min to obtain a lyophilized product; at this time, the chamber in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com