Method for purifying industrial manganese sulfate through cooperation of concentrated sulfuric acid and absolute ethyl alcohol
An anhydrous ethanol, industrial sulfuric acid technology, applied in manganese sulfate and other directions, can solve the problems of high production cost, complex process, environmental pollution, etc., and achieve the effects of less environmental pollution, simple operation, and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
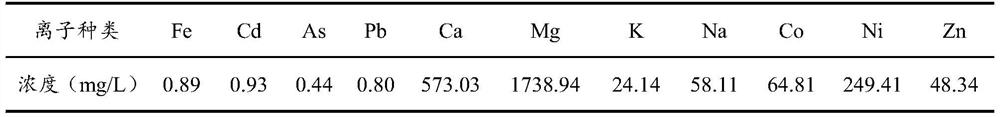
[0035] Purify the manganese sulfate solution obtained from the manganese ore leached by the sulfuric acid method in Chongzuo Branch of CITIC Dameng Mining Co., Ltd. The manganese ion content in the manganese sulfate solution is 130g / L, and other ion concentrations are shown in Table 1.
[0036] Other ion concentration in table 1 manganese sulfate solution
[0037]
[0038] The purification method is as follows:
[0039] (1) Add 50 mL of industrial manganese sulfate solution to the beaker, add 6 mL of concentrated sulfuric acid with a concentration of 98 wt% at room temperature at 25 ° C, and stir for 3 hours at a rate of 300 rpm while adding dropwise, precipitate manganese sulfate crystals, and leave it at room temperature Filter after 24 hours;
[0040](2) add deionized water to gained manganese sulfate crystal, be mixed with saturated manganese sulfate solution;
[0041] (3) adding 6mL concentration to the saturated manganese sulfate solution is the concentrated sulfuri...
Embodiment 2
[0045] The industrial manganese sulfate solution that element content is as shown in table 1 is purified, and method is as follows:
[0046] (1) Add 50 mL of industrial manganese sulfate solution into a beaker, add 9 mL of concentrated sulfuric acid with a concentration of 98 wt% at room temperature at 25 ° C, and stir for 0.5 h at a rate of 200 rpm while adding dropwise, manganese sulfate crystals are precipitated, and left to stand at room temperature Filter after 12 hours;
[0047] (2) add deionized water to gained manganese sulfate crystal, be mixed with saturated manganese sulfate solution;
[0048] (3) adding 9mL concentration to the saturated manganese sulfate solution is the concentrated sulfuric acid of 98wt%, repeat steps (1) and (2) 3 times, obtain the manganese sulfate crystal of multiple crystallization;
[0049] (4) Add 18 mL of absolute ethanol to the multi-crystallized manganese sulfate crystals, stir and wash twice, filter, and dry the obtained solid in an ov...
Embodiment 3
[0052] The industrial manganese sulfate solution that element content is as shown in table 1 is purified, and method is as follows:
[0053] (1) Add 50 mL of industrial manganese sulfate solution into a beaker, add 15 mL of concentrated sulfuric acid with a concentration of 98 wt% at room temperature at 25° C., and stir for 0.2 h at a speed of 800 rpm while adding dropwise, manganese sulfate crystals are precipitated, and left to stand at room temperature filter after 1 hour;
[0054] (2) add deionized water to gained manganese sulfate crystal, be mixed with saturated manganese sulfate solution;
[0055] (3) adding 15mL concentration to the saturated manganese sulfate solution is the concentrated sulfuric acid of 98wt%, repeat steps (1) and (2) 4 times, obtain the manganese sulfate crystal of multiple crystallization;
[0056] (4) Add 20 mL of absolute ethanol to the multi-crystallized manganese sulfate crystals, stir and wash twice, filter, and dry the obtained solid in an o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com