Yttrium-manganese-nickel perovskite structure catalyst for hydrogen production by autothermal reforming of acetic acid
A perovskite structure, autothermal reforming technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, hydrogen, etc., can solve the problem of catalyst deactivation, poor stability, poor sintering resistance and other problems, to achieve the effect of improving catalytic activity, stable activity and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Weigh 2.348g of Ni (NO 3 ) 2 ·6H 2 O, 1.237g of Y (NO 3 ) 3 ·6H 2 O and 10.982 g of 50 wt% Mn (NO 3 ) 2 The solution was transferred into a beaker containing 50 mL of ethylene glycol monomethyl ether, and stirred until the solid particles were completely dissolved; this solution was transferred into a high-pressure reactor with a polytetrafluoroethylene lining, and was placed in an oven at 200 ° C for 12 After the autoclave was cooled to room temperature, the precipitate was collected by filtration and washed three times with deionized water, and then the precipitate was placed in an oven at 105 °C to dry for 12 hours; the sample was placed in a tube furnace at 10 °C / The heating rate of min was increased to 850 °C, and the catalyst CDUT-NMY-1 was obtained after calcination at this temperature for 4 hours. The catalyst molar composition is (NiO) 1.25 (MnO 2 ) 4.75 (YO 1.5 ) 0.5 , the weight percent composition in terms of oxides is as follows: nickel oxide i...
Embodiment 1
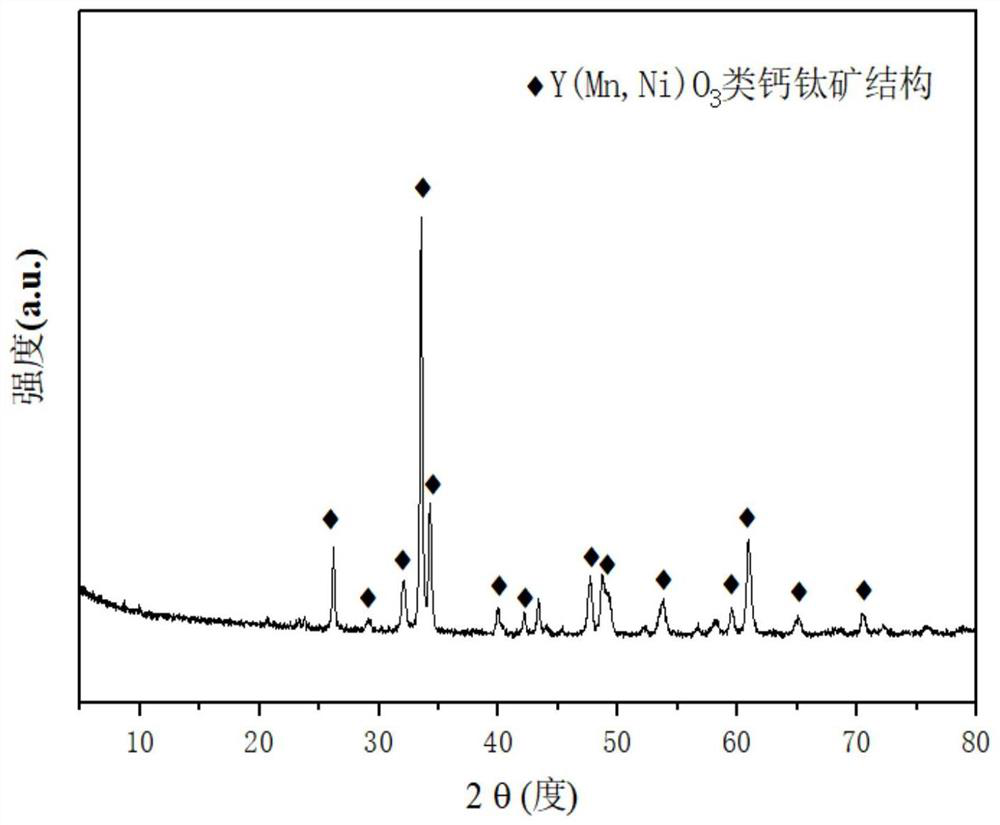
[0032] Weigh 2.326g of Ni (NO 3 ) 2 ·6H 2 O, 3.927g of Y (NO 3 ) 3 ·6H 2 O and 4.477 g of 50 wt% Mn (NO 3 ) 2 , transferred into a beaker containing 50 mL of ethylene glycol monomethyl ether, and stirred until the solid particles were completely dissolved; this solution was transferred into a high-pressure reaction kettle with a polytetrafluoroethylene lining, and placed in an oven at 200 ° C for 12 hours ; After the autoclave was cooled to room temperature, the precipitate was collected by filtration and washed three times with deionized water, and then the precipitate was placed in an oven at 105°C for drying for 12 hours; the sample was placed in a tube furnace at 10°C / min. The heating rate of the catalyst was increased to 850 ° C, and the catalyst CDUT-NMY-2 of the present invention was obtained after calcining at this temperature for 4 hours, which formed Y(Mn,Ni)O of yttrium-nickel perovskite partially substituted by manganese. 3 Perovskite-like structure catalyst...
Embodiment 2
[0035] Weigh 2.328g of Ni (NO 3 ) 2 ·6H 2 O, 2.100g of Y (NO 3 ) 3 ·6H 2 O and 8.909 g of 50 wt% Mn (NO 3 ) 2 , transferred into a beaker containing 50 mL of ethylene glycol monomethyl ether, and stirred until the solid particles were completely dissolved; this solution was transferred into a high-pressure reaction kettle with a polytetrafluoroethylene lining, and placed in an oven at 200 ° C for 12 hours ; After the autoclave was cooled to room temperature, the precipitate was collected by filtration and washed three times with deionized water, and then the precipitate was placed in an oven at 105°C for drying for 12 hours; the sample was placed in a tube furnace at 10°C / min. The heating rate was increased to 850 °C, and after calcination at this temperature for 4 hours, the catalyst CDUT-NMY-3 was obtained, forming a yttrium-nickel-based perovskite catalyst partially substituted by manganese. The catalyst molar composition is (NiO) 0.73 (MnO 2 ) 2.27 (YO 1.5 ) 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
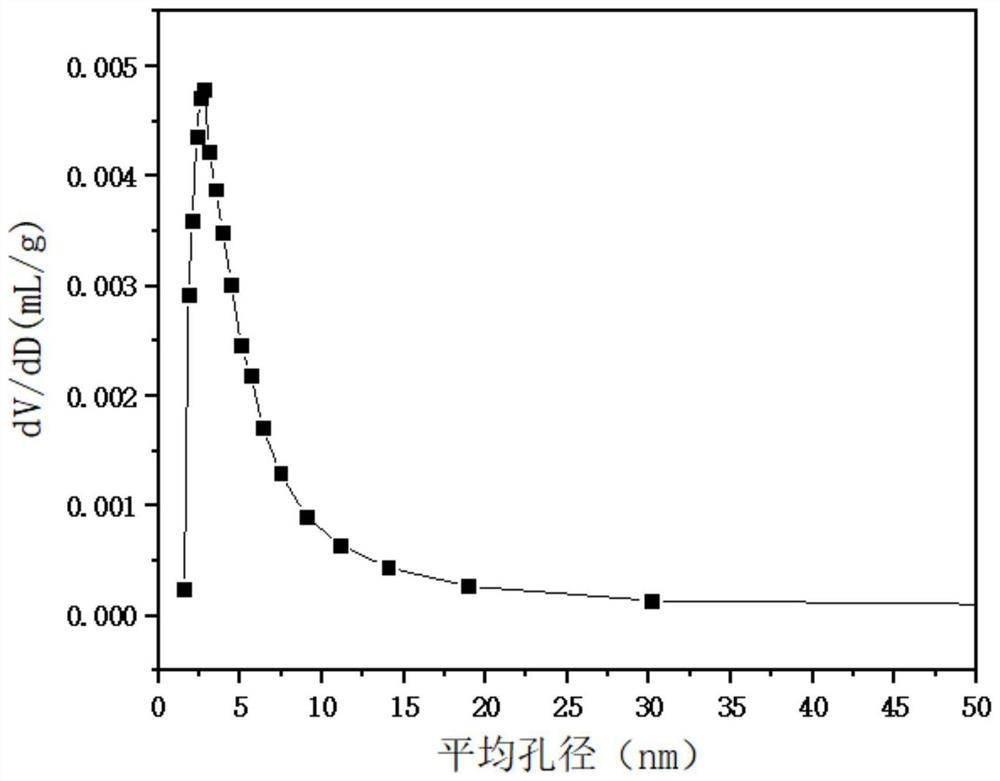
| Pore volume | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com