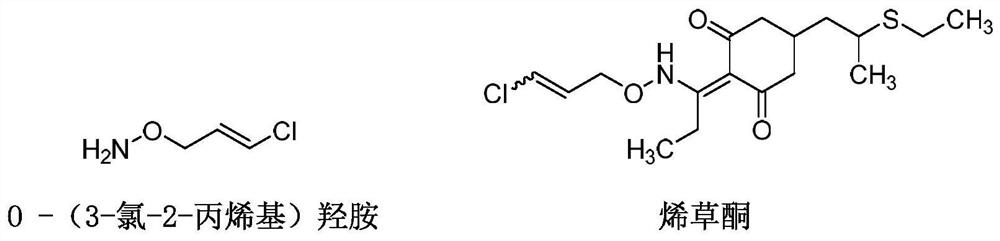
Synthesis method of O-(3-chloro-2-propenyl) hydroxylamine
A synthetic method and propylene-based technology, applied in the direction of hydroxylamine, chemical instruments and methods, nitrogen compounds, etc., can solve the problems of large amount of three wastes, low production efficiency, inconvenient industrialization, etc., to reduce salt content, improve product yield and quality, and the effect of reducing the cost of wastewater treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
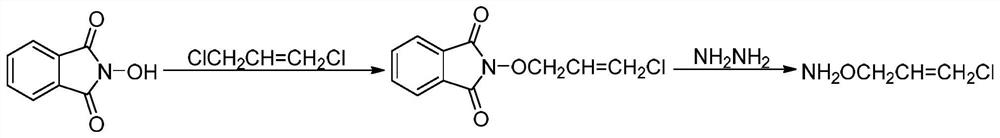
[0031] The invention provides a kind of synthetic method of O-(3-chloro-2-propenyl) hydroxylamine, comprises the steps:
[0032] (1) Treat an aqueous solution of hydroxylamine salt (hydroxylamine hydrochloride or hydroxylamine sulfate) with a basic ion exchange resin to obtain a hydroxylamine solution. The reaction formula is:
[0033]
[0034] (2) After preheating the hydroxylamine solution and methyl isobutyl ketone, pump them into the first-stage microchannel reactor respectively, and react to obtain methyl isobutyl ketone oxime, and then the reaction solution flowing out of the microchannel reactor directly enters the first-stage microchannel reactor. In the secondary microchannel reactor, continue to react with 1,3-dichloropropene and liquid caustic soda. After the reaction, the reaction solution is adjusted to weak acidity with acid, and the phases are separated. The aqueous phase is extracted with methyl isobutyl ketone and combined. The organic phase is washed with...
Embodiment 1
[0050] The synthetic method of O-(3-chloro-2-propenyl) hydroxylamine in the present embodiment comprises the steps:
[0051] Hydroxylamine hydrochloride (70.0g, 1mol) aqueous solution is passed through the 717 strong basic styrenic anion exchange resins that have been processed, and the effluent liquid is pumped in the first-stage microchannel reactor at room temperature, and simultaneously pumps into methyl isobutyl ketone ( 150g, 1.5mol), react, and the reaction temperature is controlled at 80-100 ℃; The effluent of the first stage microchannel reactor directly enters in the second stage microchannel reactor, and simultaneously adds 1,3-dichloropropene (116.6 g, 1.05mol), 30% sodium hydroxide (147g, 1.1mol) solution, continue the reaction, and the reaction temperature is controlled at 80-90°C; after the reaction, the phases are separated, and the aqueous phase is extracted with methyl isobutyl ketone (50mL × 3), the organic phase and the extraction phase were combined, washe...
Embodiment 2
[0054] The synthetic method of O-(3-chloro-2-propenyl) hydroxylamine in the present embodiment comprises the steps:
[0055] Hydroxylamine hydrochloride (70.0g, 1mol) aqueous solution is passed through the 717 strong basic styrenic anion exchange resins that have been processed, and the effluent liquid is pumped in the first-stage microchannel reactor at room temperature, and simultaneously pumps into methyl isobutyl ketone ( 150g, 1.5mol), react, and the reaction temperature is controlled at 20-50 ℃; The effluent of the first-stage microchannel reactor directly enters in the second-stage microchannel reactor, and simultaneously adds 1,3-dichloropropene (116.6 g, 1.05mol), 30% sodium hydroxide (147g, 1.1mol) solution, continue to react, the reaction temperature is controlled at 50-60 ℃; × 3), the organic phase and the extraction phase were combined, washed with water and then azeotropically dewatered, and the solvent was recovered to obtain 100.8 g of O-(3-chloro-2-propenyl) h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com