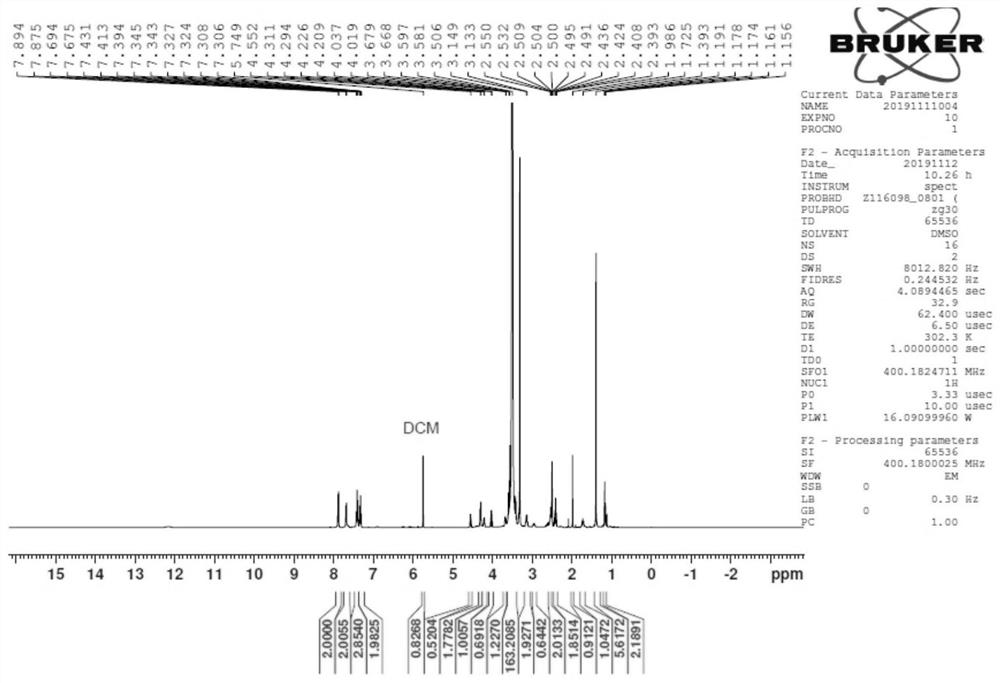
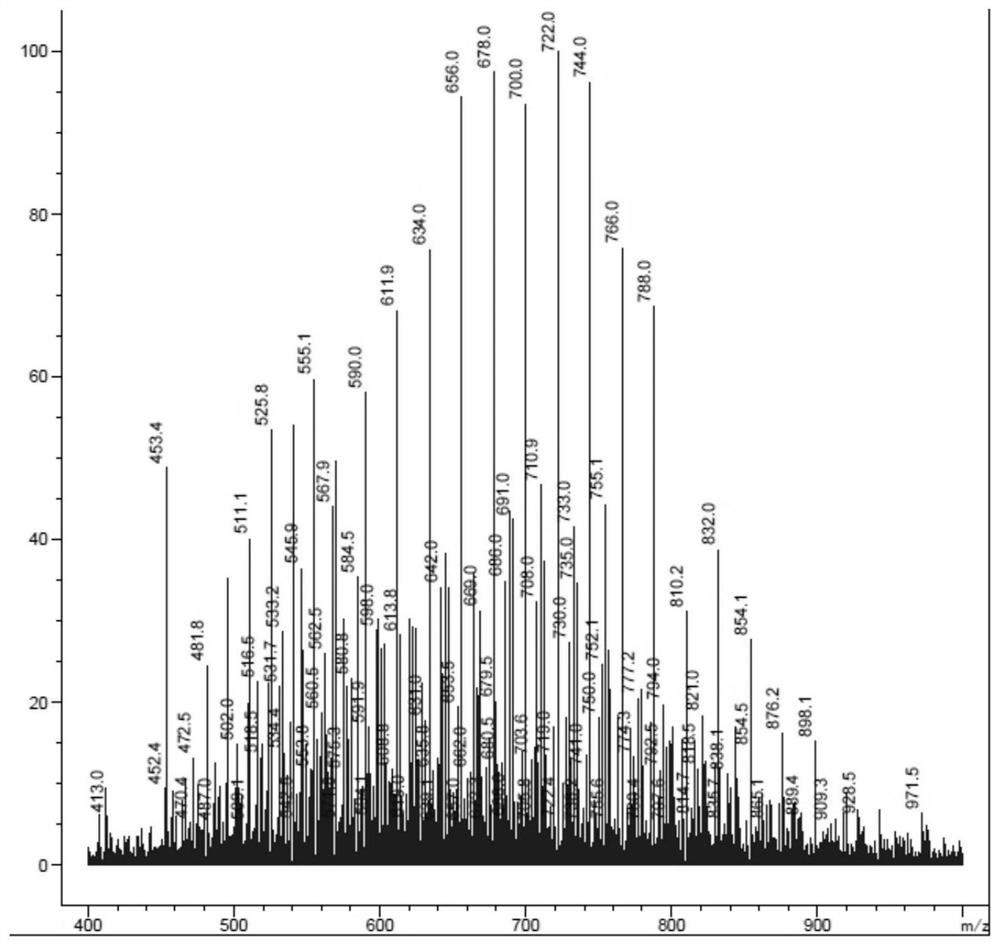
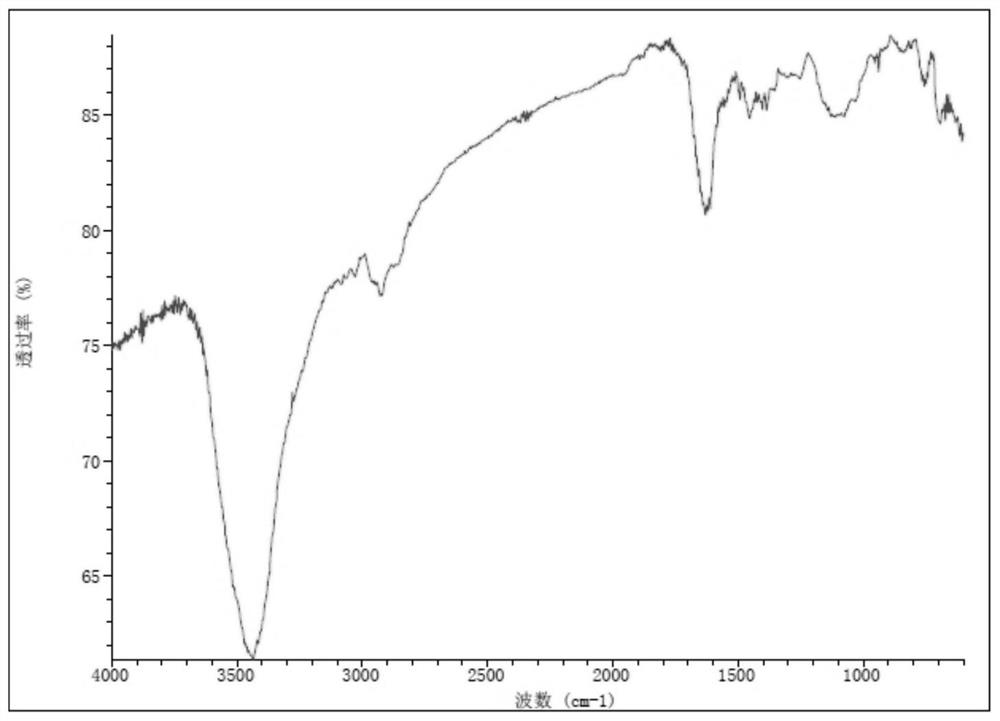
Sulfur-containing polyethylene glycol resins for polypeptide synthesis
A technology for synthesis of polyethylene glycol and peptides, which is applied in the preparation method of peptides, peptides, organic chemistry, etc., can solve the problems of polyethylene glycol disorder, large swelling, unevenness, etc., and achieve stable chemical properties and quality , The effect of increasing the coupling speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Example 1H 2 N-PEG (60~80) -CH 2 CH 2 CH 2 -S-CH 2 CH 2 -COOH (compound 7, R 3 =H)
[0192] 1) HO-PEG (60~80) -CH 2 CH 2 CH 2 -S-CH 2 CH 2 -COOtBu synthesis
[0193] Dissolve 840g (about 0.24mol) of APEG3500 in 7L of 1,4-dioxane, under nitrogen protection, add 88.9g (0.6mol) of tert-butyl 2-mercaptoacetate, raise the temperature to 55°C, and add 9.4g of (0.03mol) AIBN, when the temperature stabilized to 55°C, remove the nitrogen protection, react at 55°C for 13h, and MS detected that the reaction was complete.
[0194] About 4L of 1,4-dioxane was removed by rotary evaporation under reduced pressure, 10L of water was added, and petroleum ether (5L×3) was used to extract a large amount of unreacted tert-butyl 2-mercaptoacetate. The aqueous layer was adjusted to pH=4 with 40% phosphoric acid (a small amount of acid is enough), extracted with dichloromethane (5L*2), washed once with water, dried with anhydrous sodium sulfate, and evaporated to dryness under r...
Embodiment 2
[0204] Example 2 Me-HN-PEG (8~12) -CH 2 CH 2 CH 2 -S-CH 2 CH 2 COOH (compound 9, R 3 = Hydrogen, R 5 = methyl)
[0205] 1) Me-NH-PEG (8~12) -CH 2 CH 2 CH 2 -S-CH 2 CH 2 -COOtBu
[0206] 70g (0.1mol) Tos-O-PEG (8~12) -CH 2 CH 2CH 2 -S-CH 2 CH 2 -COOtBu was dissolved in 400ml of tetrahydrofuran, cooled to -60°C, and 100ml of 3M methylamine tetrahydrofuran solution was added dropwise. After the addition was complete, it was kept at -60°C and stirred for 3 hours. The solvent was evaporated to dryness under reduced pressure to obtain a pale yellow oil, 400ml of water was added to dissolve the oil, the aqueous phase was extracted with ethyl acetate (200ml×3), the acetic acid was washed once with 200ml of saturated brine, dried over anhydrous sodium sulfate, and evaporated under reduced pressure. On drying, 53 g of a yellow oil were obtained.
[0207] 2) Me-NH-PEG (8~12) -CH 2 CH 2 CH 2 -S-CH 2 CH 2 -COOH
[0208] 35g (52mmol) NH 2 -PEG (20~30) -CH 2 CH...
Embodiment 3
[0211] NH 2 -CH 2 -CH 2 -S-CH 2 CH 2 CH 2 -PEG(30~40)-CH 2 CH 2 COOH (Compound 16, R1=H, R2=H)
[0212] 1) Synthesis of Boc-NH-CH2-CH2-S-CH2CH2CH2-PEG(30~40)-OH (compound 14)
[0213] Dissolve 150g (about 0.1mol) of APEG1500 in 600ml of 1,4 dioxane, under nitrogen protection, add 106g (0.6mol) of Boc-cysteamine (compound 13), heat up to 55°C, add 3.3g of ( 0.02mol) AIBN, when the temperature stabilized to 55°C, remove the nitrogen protection, react at 55°C for 9h, and MS detected that the reaction was complete.
[0214] Remove about 400ml of 1,4-dioxane by rotary evaporation under reduced pressure, add 400ml of water, and extract a large amount of unreacted Boc-cysteamine with petroleum ether (400ml×3). The aqueous layer was adjusted to pH=4 with 40% phosphoric acid, extracted with dichloromethane (300ml×2), washed once with water, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain Boc-NH-CH 2 -CH 2 -S-CH 2 CH 2 CH 2 -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com