System and method for high-selectivity preparation of xylene from methanol and oriented conversion of xylene isomer
A directional transformation and high selectivity technology, applied in chemical instruments and methods, isomerization hydrocarbon production, catalysts, etc., can solve the problems of complex reaction process, low xylene selectivity, and low comprehensive utilization rate of by-products, and achieve Improve selectivity and yield, ensure conversion and utilization, and improve the effect of comprehensive utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
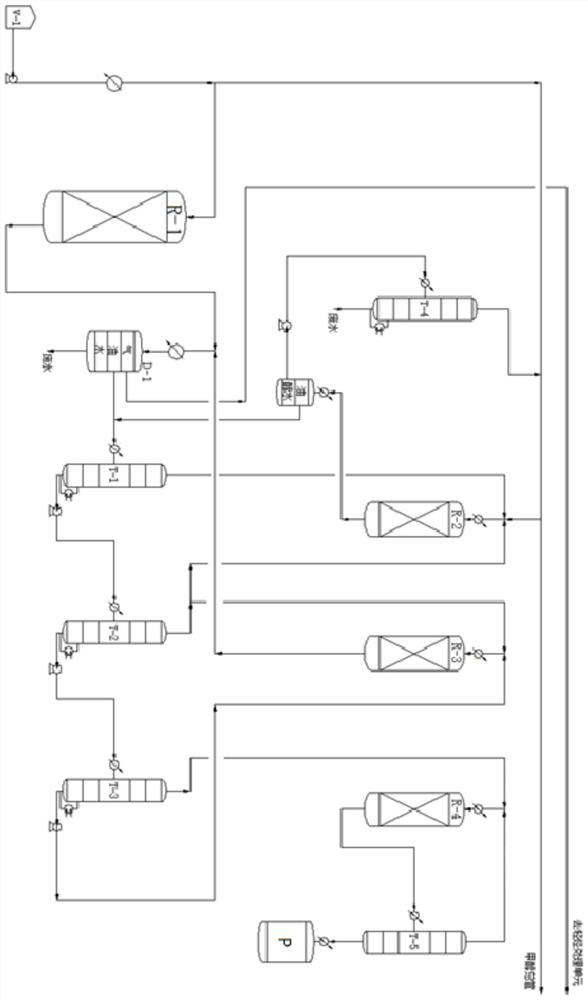
[0066] Such as figure 1 Shown, a kind of system that is used for methanol highly selective preparation xylene and xylene isomer directional conversion, system comprises following device:
[0067] A methanol aromatization reaction unit (R-1), which is used to perform an aromatization reaction on the raw material methanol to generate an aromatization product, and the aromatization product contains an aromatic hydrocarbon mixture of benzene, toluene, xylene and / or trimethylbenzene;
[0068] The three-phase separation unit (D-1) is used to separate the aromatized product extracted from the methanol aromatization reaction unit into an aromatized gas phase product, an aromatized oil phase product and an aromatized water phase product;
[0069] Debenzene tower unit (T-1), detoluene tower unit (T-2) and dexylene tower unit (T-3), debenzene tower unit (T-1), detoluene tower unit (T-2) It is connected in series with the de-xylene tower unit (T-3) in sequence, and is used to send the ar...
Embodiment 2
[0094] A system for producing xylene with high selectivity of methanol and directional conversion of xylene isomers is the same as in Example 1.
[0095] The specific process steps (1)-(4) with OX (o-xylene) as the target product are the same as in Example 1.
[0096] The difference is that in step (5), the xylene mixed isomers withdrawn from the top of the de-xylene tower unit (T-3) are sent to the xylene isomerization reaction unit (R-4), and the Directional conversion of mixed isomers of xylene to products containing OX (orth-xylene isomer). Subsequently, the product containing OX generated by the xylene isomerization reaction unit (R-4) is sent to the xylene separation tower unit (T-5), the product in the tower is OX, and the top product is returned to the xylene isomerization Reaction unit (R-4);
[0097] The xylene isomerization reaction unit (R-4) adopts a fixed bed reactor, the reaction temperature is 450°C, the reaction pressure is normal pressure, and the mass spac...
Embodiment 3
[0099] A system for producing xylene with high selectivity of methanol and directional conversion of xylene isomers is the same as in Example 1.
[0100] The specific process steps (1)-(4) with MX (m-xylene) as the target product are the same as in Example 1.
[0101] The difference is that in step (5), the xylene mixed isomers withdrawn from the top of the de-xylene tower unit (T-3) are sent to the xylene isomerization reaction unit (R-4), and the Directional conversion of mixed isomers of xylene to products containing MX (m-xylene isomer). Subsequently, the product containing MX generated by the xylene isomerization reaction unit (R-4) is sent to the xylene separation tower unit (T-5), the product in the tower is MX, and the overhead product is returned to the xylene isomerization Reaction unit (R-4);
[0102] The xylene isomerization reaction unit (R-4) adopts a fixed bed reactor, the reaction temperature is 450°C, the reaction pressure is normal pressure, and the mass sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com