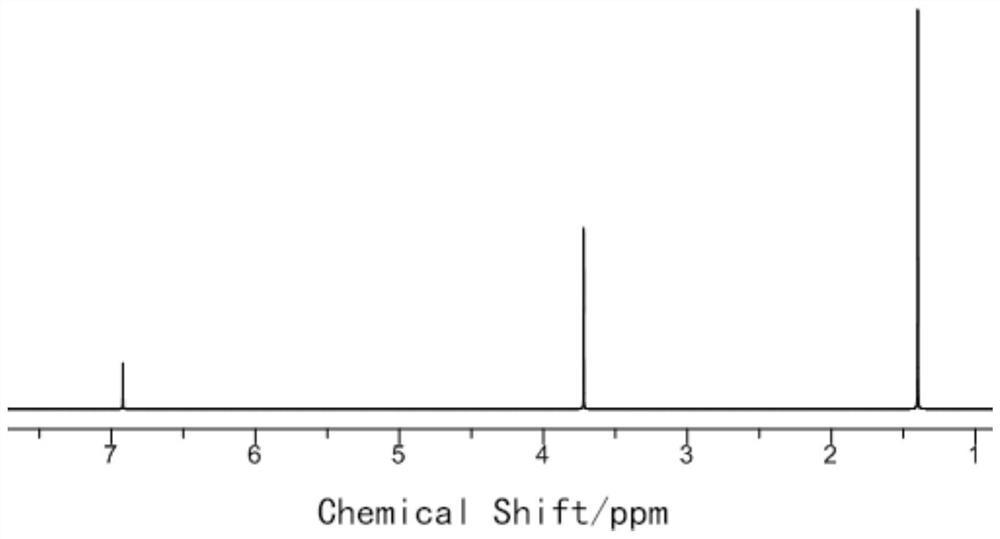
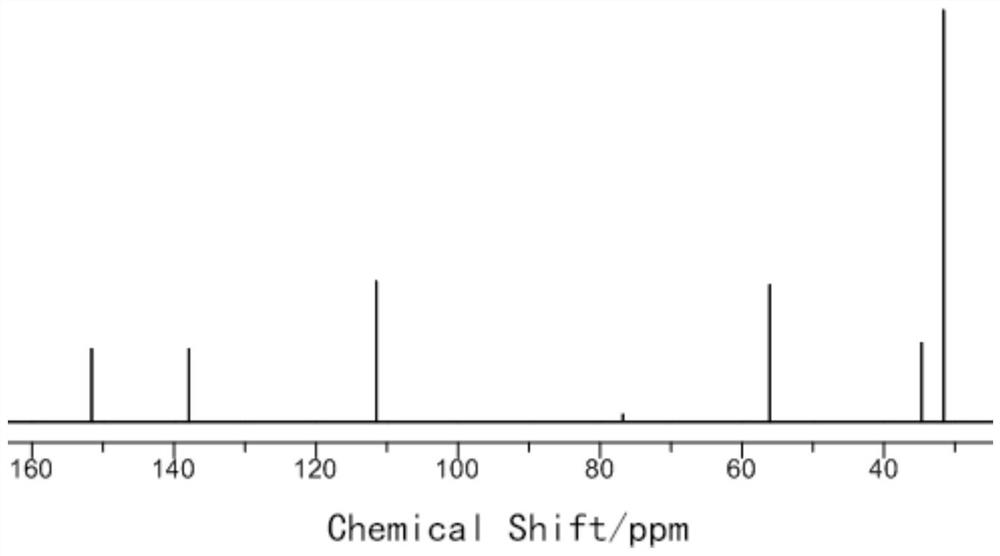
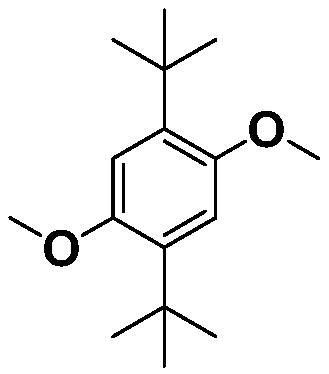
Preparation method of 2, 5-di-tert-butyl-p-dimethoxybenzene
A technology of di-tert-butyl-p-phthalene and di-tert-butyl-hydroquinone, which is applied in the field of preparation of 2,5-di-tert-butyl-p-xylylene, can solve the problem of inconvenient operation in the production process, Long production process, many wastes, etc., to achieve the effect of low cost, less reaction steps, and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of 2,5-di-tert-butylhydroquinone
[0035] Take 55.0g (0.5mol) of hydroquinone, put it into a three-necked flask, add 40g of deionized water into the three-necked flask, stir and heat to 80°C, take 60g (0.81mol) of tert-butanol, and slowly drop it into the three-necked flask, Until the solid in the bottle is completely dissolved, take 163g (2.2mol) tert-butanol and 10g sulfuric acid to make a mixed solution, slowly drop it into a three-necked flask, keep the reaction temperature at 80°C, and react for 3 hours. After the reaction is completed, cool the system to room temperature. After filtering, the obtained solid was washed three times with 150 ml of water and dried to obtain 107.9 g of 2,5-di-tert-butylhydroquinone with a yield of 97%.
[0036] (2) Preparation of 2,5-di-tert-butyl-p-xylylene ether
[0037] Take 44.5g (0.2mol) of 2,5-di-tert-butylhydroquinone prepared in step (1) and dissolve in 500g DMF, add 0.46g (19mmol) sodium hydride under nitrogen...
Embodiment 2
[0039] (1) Preparation of 2,5-di-tert-butylhydroquinone:
[0040] Take 110.0g (1.0mol) of hydroquinone, put it into a three-necked flask, add 80g of deionized water into the three-necked flask, stir and heat to 70°C, take 90g (1.2mol) of tert-butanol, and slowly drop it into the three-necked flask, Until the solid in the bottle is completely dissolved, take 244.5g (3.3mol) of tert-butanol and 4g of 36.5% hydrochloric acid by mass to form a mixed solution, slowly drop it into a three-necked flask, keep the reaction temperature at 70°C, and react for 2 hours. After the completion, the system was cooled to room temperature, filtered, and the obtained solid was washed three times with 200 ml of water, and dried to obtain 2,5-di-tert-butylhydroquinone (200 g, yield 90%).
[0041] (2) Preparation of 2,5-di-tert-butyl-p-phthalyl ether:
[0042] Take 89g (0.4mol) of 2,5-di-tert-butylhydroquinone prepared in step (1) and dissolve in 500g of dimethyl carbonate, add 1.0g (25mmol) of potas...
Embodiment 3
[0044] (1) Preparation of 2,5-di-tert-butylhydroquinone:
[0045] Take 55.0g (0.5mol) of hydroquinone, put it into a three-necked flask, add 40g of deionized water into the three-necked flask, stir and heat to 90°C, take 30g (0.4mol) of tert-butanol, and slowly drop it into the three-necked flask, Until the solid in the bottle is completely dissolved, take 81.5g (1.1mol) of tert-butanol and 0.3g of sulfuric acid to form a mixed solution, slowly drop it into a three-necked flask, maintain the reaction temperature at 90°C, and react for 5h. After the reaction is completed, the system is cooled to After filtering at room temperature, the obtained solid was washed three times with 150 ml of water and dried to obtain 97.9 g of 2,5-di-tert-butylhydroquinone with a yield of 88%.
[0046] (2) Preparation of 2,5-di-tert-butyl-p-phthalyl ether:
[0047] Dissolve 89g (0.4mol) of 2,5-di-tert-butylhydroquinone prepared in step (1) in 800g THF, add 0.17g (4mmol) calcium hydride under nitro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com