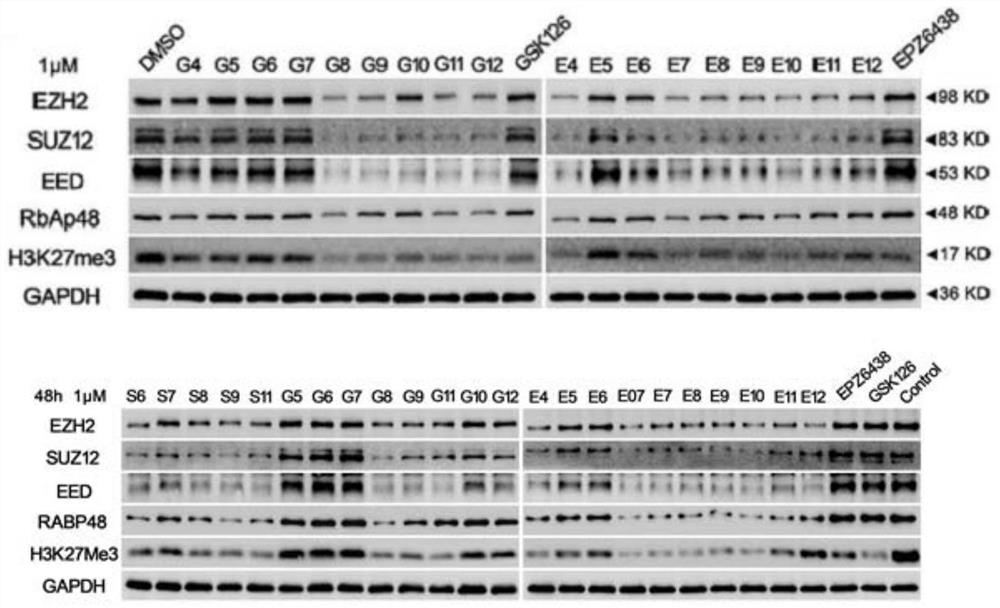
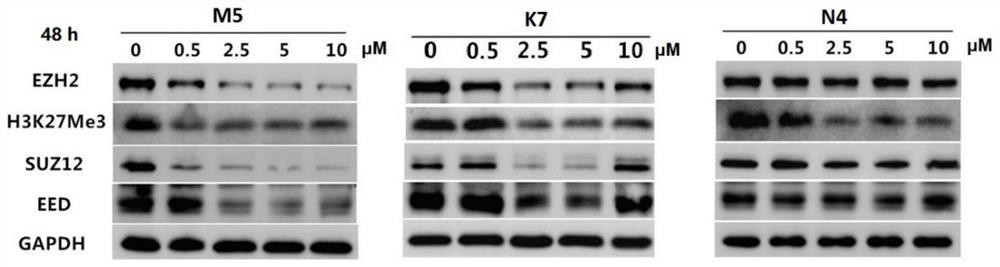
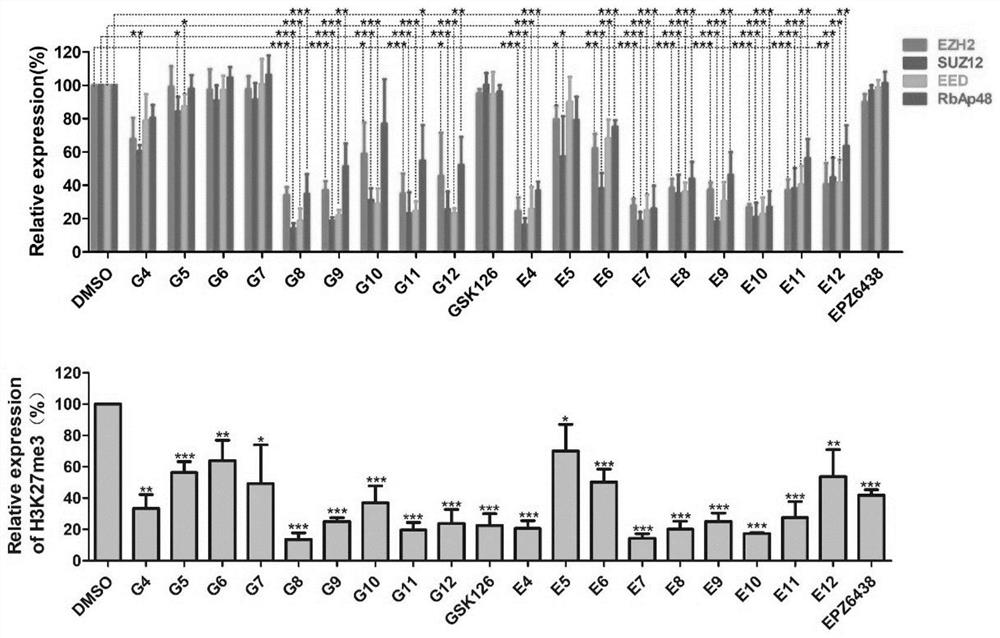
Bifunctional compound capable of inducing PRC2 protein complex core subunit degradation, pharmaceutical composition and application
A compound, bifunctional technology, applied in the field of chemical medicine, can solve the problems of affecting the catalytic activity of HDAC, unable to effectively inhibit EZH2, unable to degrade various core subunits, etc., and achieve good anticancer activity, low toxicity, and good antitumor activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] This example provides the synthesis and related chemical data of nine bifunctional compounds G4-G12. The synthetic route of G4-G12 is as follows:
[0065]
[0066] The specific preparation process is:
[0067] The first step: preparation 1b, ammonolysis reaction.
[0068] 1a (3.28g, 20mmol, 1.0eq), 3-aminopiperidine-2,6-dione (3.1g, 24mmol, 1.2eq) was dissolved in 50mL of acetic anhydride, heated to reflux at 140°C for 6h, and decompressed after the reaction was completed The reaction solvent was removed by distillation, and the residue was poured into water, and a light gray precipitate was precipitated, filtered with suction, the filter cake was washed with water, and dried to obtain product 1b (3.84g, 71%). HRMS m / z calculated for C13H10N2O5[M+H]+: 275.0662, found: 275.0686.
[0069] The second step: 1c-1k general synthesis steps.
[0070] 1b (1mmol, 1.0eq), DIPEA (3mmol, 3.0eq) and dibromoalkane (1.2mmol, 1.2eq) were dissolved in DMF (10mL), and reacted at 85-1...
Embodiment 2
[0110] This example provides the synthesis and related chemical data of nine bifunctional compounds E4-E12. The synthetic route of E4-E12 is as follows:
[0111]
[0112] The specific preparation process is:
[0113] The first step: 2-methyl-3-bromo-5-nitrobenzoic acid methyl ester 2a (5.5g, 20mmol, 1.0eq), ammonium chloride (5.6g, 100mmol, 5.0eq) was dissolved in aqueous ethanol ( 60mL, H 2 O:EtOH=1:3), after heating up to 80°C, iron powder (11.2g, 200mmol, 10.0eq) was added three times. Thin-layer chromatography (TLC) detection reaction is completed after reacting 1h, and diatomaceous earth filter aids, and filtrate decompression distills off solvent, and residue is extracted through DCM, and Na 2 SO 4 Concentration after drying afforded product 2b (4.41 g, 92%) without further purification. 1 H NMR (400MHz, CDCl 3 )δ7.31(d,J=2.6Hz,1H),6.92(d,J=2.6Hz,1H),3.87(s,3H),3.82(s,2H),2.26(s,3H).HRMS m / z calculated for C 9 h 10 BrNO 2 [M+H] + :243.9967,found:243.9958. ...
Embodiment 3
[0149] This example provides the synthesis and related chemical data of nine bifunctional compounds S4-S12. The synthetic route of S4-S12 is as follows:
[0150]
[0151] The specific preparation process is:
[0152] The first step: S1 (4mmol) and S2 (4.4mmol) were dissolved in DMSO (10mL), HOAT (0.55g, 1.5mmol) and EDCI (0.84g, 2.2mmol) were added, and the reaction solution was stirred at 45°C for 20h. After the completion of the reaction monitored by TLC, the reaction solution was poured into ice water (100mL), stirred for 30min, precipitated out, filtered, washed with water, dried, dissolved in a mixture of methanol and chloroform (10:1), mixed, passed through a silica gel column layer Analysis and purification afforded yellow solid S3 (1.57 g). 1 H NMR (400MHz, Chloroform-d) δ12.78(s, 1H), 7.41(t, J=5.7Hz, 1H), 7.22(d, J=2.0Hz, 1H), 7.18(d, 2.0Hz, 1H ), 4.54(d, J=5.7Hz, 2H), 3.94(dt, J=11.6, 3.3Hz, 2H), 3.36–3.25(m, 2H), 3.02(q, J=7.0Hz, 2H), 2.95 (m,1H),2.93(dd,J=7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com