Method for detecting lincomycin impurity F in lincomycin hydrochloride injection by high performance liquid chromatography-evaporative light method
A technology of high-performance liquid chromatography and lincomycin hydrochloride, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of inability to accurately identify and detect impurity F, quantitative detection, inability to separate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The detection method of the present invention is used to detect lincomycin impurity F in lincomycin hydrochloride injection, wherein the chromatographic conditions for detection are evaporative light scattering detector drift tube temperature 50°C; carrier gas flow rate is 1.5L / min; The temperature is 30°C; the flow rate is 0.9ml / min.
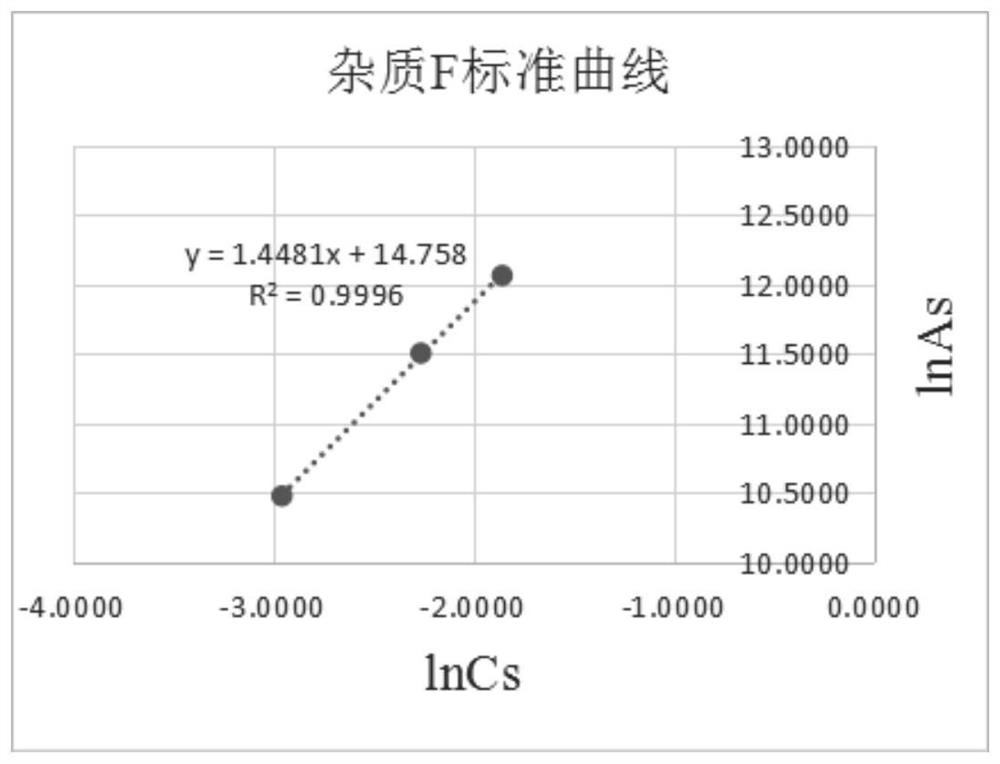
[0041] The detection data of each concentration gradient of lincomycin impurity F standard substance are shown in Table 1:
[0042] Table 1 The detection data of each concentration gradient of lincomycin impurity F standard substance
[0043]
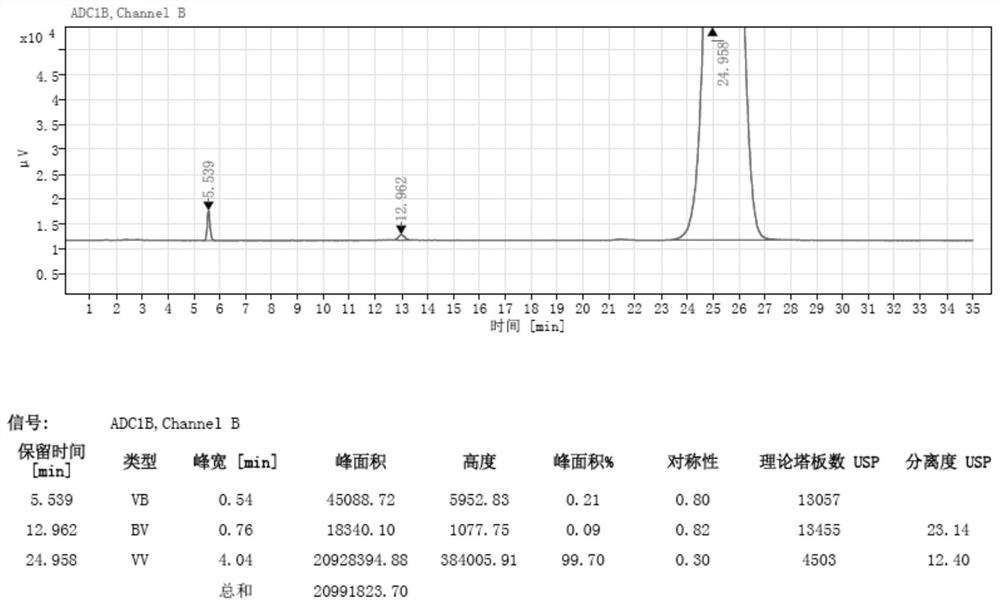
[0044] Wherein, the chromatogram of lincomycin impurity F standard substance 2 is as follows figure 1 As shown, the chromatogram of the sample solution to be tested is as follows figure 2 shown.
[0045] Draw the standard curve of lincomycin impurity F standard substance according to the detection data such as the concentration of above-mentioned standard substance and peak area, as image...
Embodiment 2
[0054] The detection method of the present invention is used to detect lincomycin impurity F in lincomycin hydrochloride injection, wherein the chromatographic conditions for detection are evaporative light scattering detector drift tube temperature of 60°C; carrier gas flow rate of 2.2L / min; The temperature is 35°C; the flow rate is 1.2ml / min.
[0055] In the second group of sample solution to be tested, the chromatogram of lincomycin impurity F is as follows Figure 4 shown.
[0056] Using the chromatographic conditions in this example, the detected peak elution time of lincomycin impurity F was 5.546 min, and the resolution was 23.77. This method can still qualitatively and quantitatively detect lincomycin impurity F.
Embodiment 3
[0058] The lincomycin impurity F standard substance and the third group of sample solutions to be tested were detected by using the high-performance liquid chromatography ultraviolet detection method recorded in the prior art "Chinese Pharmacopoeia".
[0059] Wherein, the chromatographic conditions for the ultraviolet detection of the high-performance liquid chromatograph are as follows: the detection wavelength is 214nm; the column temperature is 30°C; and the flow rate is 0.5ml / min.
[0060] The chromatogram of the lincomycin impurity F standard substance detected by high performance liquid chromatography is as follows Figure 5 As shown, the chromatograms of the third group of sample solutions to be tested are as follows Image 6 shown.
[0061] In the present embodiment, the chromatogram of lincomycin impurity F standard substance Figure 5 It shows that its peak time is 6.155min, the retention time is too short, and partially overlaps with the diluent peak; this has jus...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com