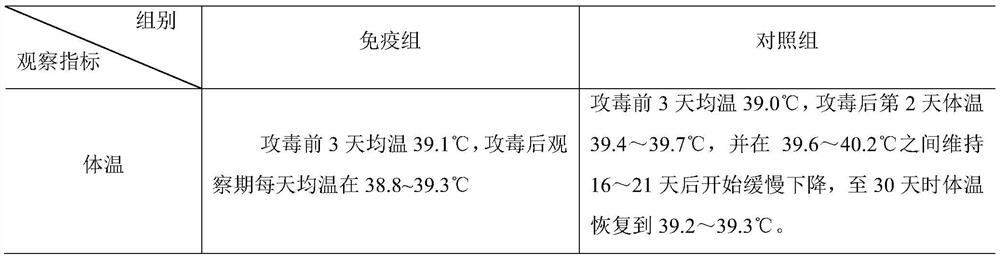
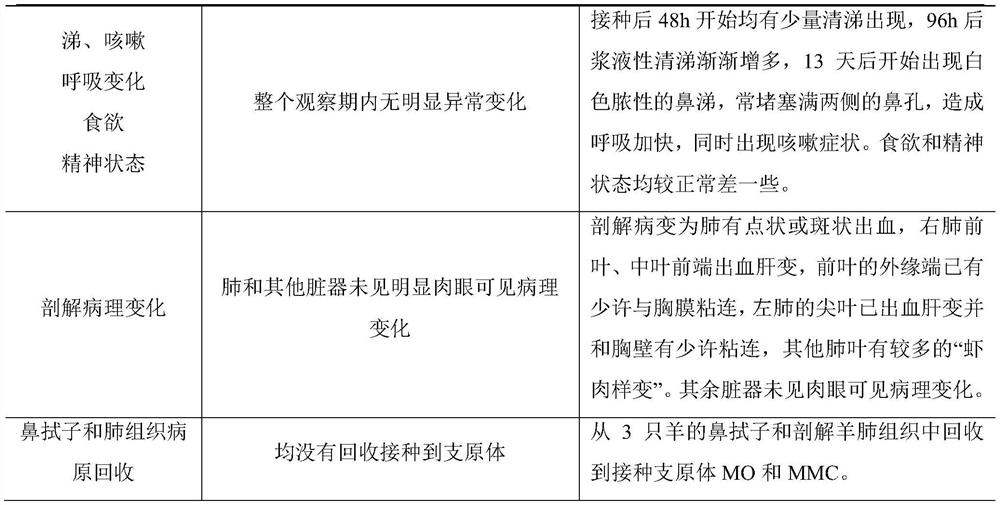
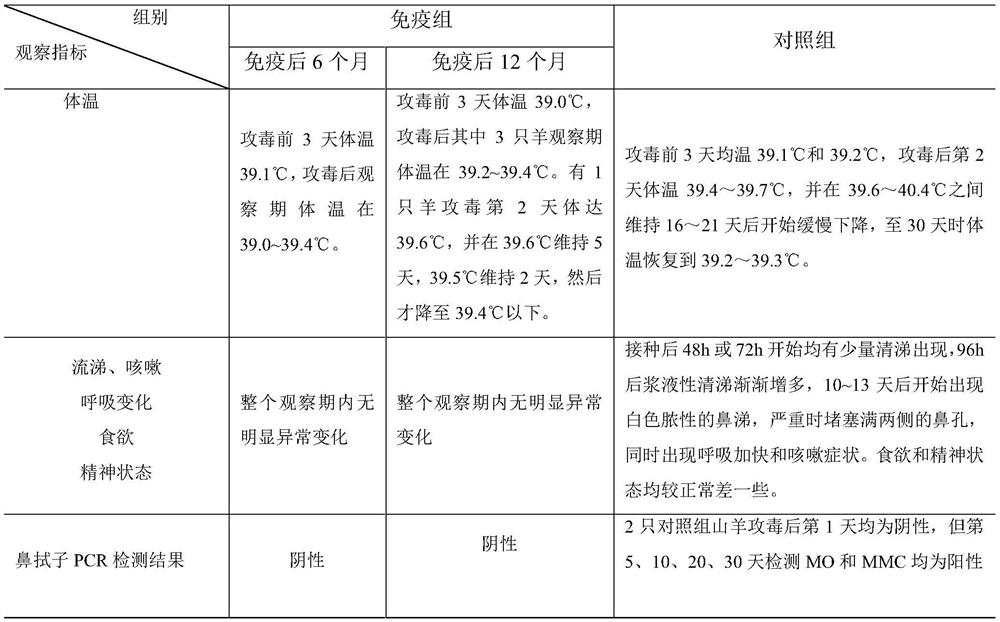
Combined inactivated vaccine for goat mycoplasma pneumonia and preparation method of combined inactivated vaccine
A dual inactivated vaccine, mycoplasma pneumonia technology, applied in biochemical equipment and methods, vaccines, multivalent vaccines, etc., can solve problems such as indistinguishable diseases, mixed infection, etc., and achieve the effect of reducing production costs and preventing infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of double inactivated vaccine of mycoplasma goat pneumoniae
[0041] 1. Preparation of mycoplasma culture medium:
[0042] (1) Preparation of pancreas extract
[0043] Take fresh pancreas from pigs that have passed the quarantine inspection, remove fat tissue, and chop; add 1500mL ddH to 500g pancreas 2 O and 500mL absolute ethanol, shake once every 2-4 hours at room temperature, and filter with filter paper after 72 hours;
[0044] (2) Preparation of Pig Lung Digestive Soup
[0045] Take the fresh pig lungs of pigs that have passed the quarantine inspection, remove the trachea and lymphatic connective tissue, chop them up, add 2000mL ddH to 1500g pig lungs 2 O, stirred and heated to 80°C, then added anhydrous NaHCO with a mass concentration of 0.8% 3 2500mL, mix well and cool to 45°C, add 500mL pancreas extract and 50mL chloroform, mix well and place at 37°C for 6-7h, then add 40mL concentrated sulfuric acid, heat at 100°C for 1h, fil...
Embodiment 2
[0058] Embodiment 2: the sterility test of the capra mycoplasma pneumonia dual inactivated vaccine
[0059] Inoculate the mycoplasma pneumoniae double inactivated vaccine prepared in Example 1 on nutrient agar medium and mycoplasma medium, at 37° C., 5% CO 2 Under culture for 3 to 7 days, no microorganisms grew.
Embodiment 3
[0060] Embodiment 3: the safety test of the double inactivated vaccine of mycoplasma goat pneumonia
[0061] (1) Material:
[0062] 2 rabbits weighing 1.5-2.0 kg.
[0063] The Mycoplasma caprico pneumonia double inactivated vaccine that embodiment 1 makes.
[0064] (2) Inspection method:
[0065] Shake the Mycoplasma caprico pneumoniae dual inactivated vaccine prepared in Example 1, inject 2 mL into each rabbit's thigh intramuscularly, raise it for 14 days under normal conditions, measure the temperature on the 1st, 5th, 10th, and 14th day after injection and Observe their food intake, mental state and whether there is any inflammatory reaction at the injection site.
[0066] (3) Test results
[0067] After the injection of the vaccine, the two rabbits had no abnormal reaction, no inflammation at the injection site, and the body temperature was 38.5-39.0°C, indicating that the prepared vaccine was safe.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com