Application of anti-EGFR scFv:: FTH1/FTH1 protein nanoparticles
A nanoparticle and protein technology, applied in the field of nanobiomedicine, can solve the problem of lack of specific targeting of triple-negative breast cancer cells, and achieve the effect of inhibiting proliferation and survival, inhibiting effect, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation and Characterization of Anti-EGFR ScFv::FTH1 / FTH1 Protein Nanoparticles
[0035] Two plasmids, pET-28(+)-FTHI and pET-28a(+)-anti-EGFR scFV-FTH1, exist in our laboratory (see ZL201010239499.3 for specific preparation methods). Wherein, the amino acid sequence of the anti-EGFR scFv::FTH1 protein is shown in SEQ ID NO:1, and the amino acid sequence of the FTH1 protein is shown in SEQ ID NO:2. Escherichia coli competent cells E.coli.BL21(DE3) and E.coli.DH5a were purchased from Beijing Tiangen Biochemical Technology Company. The above two plasmids were transformed into E.coli.DH5a competent cells, and the positive clones were screened by colony PCR and enzyme digestion identification. Afterwards, the expression vector with the correct sequence was transformed into Ecoil.BL.21(DE3) competent cells to obtain engineering expression strain cells containing FTH1 protein gene target engineering expression strain cells and anti-EGFR scFv::FTH1 protein gene ...
Embodiment 2
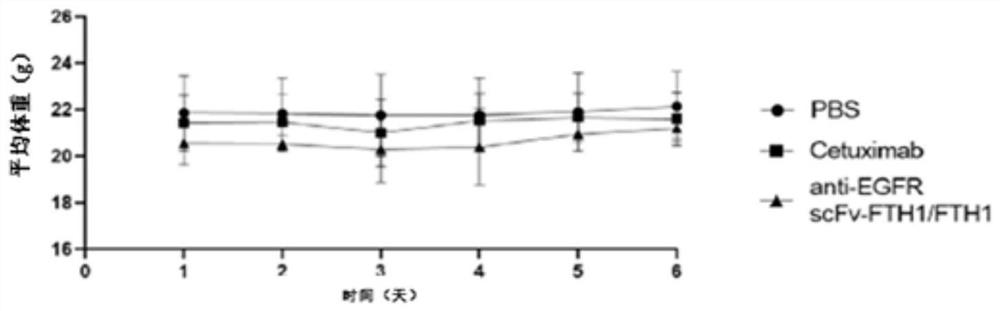
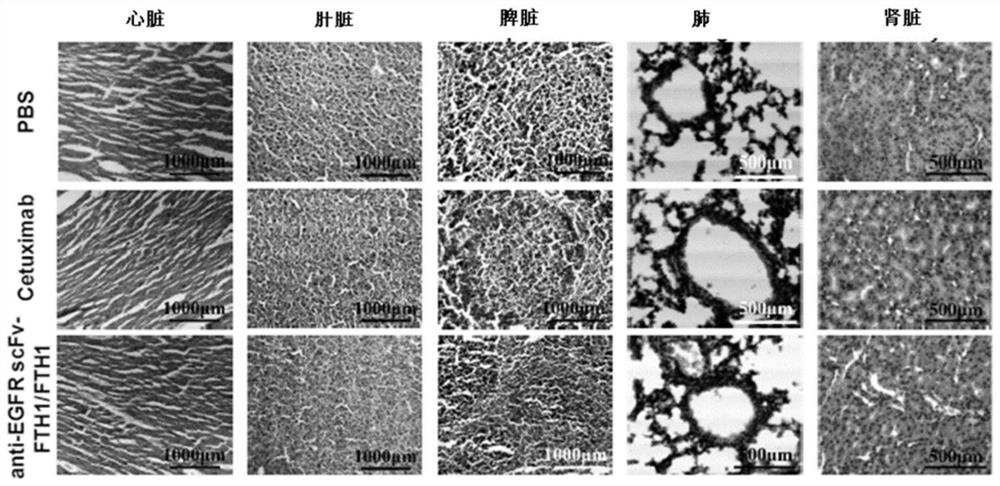
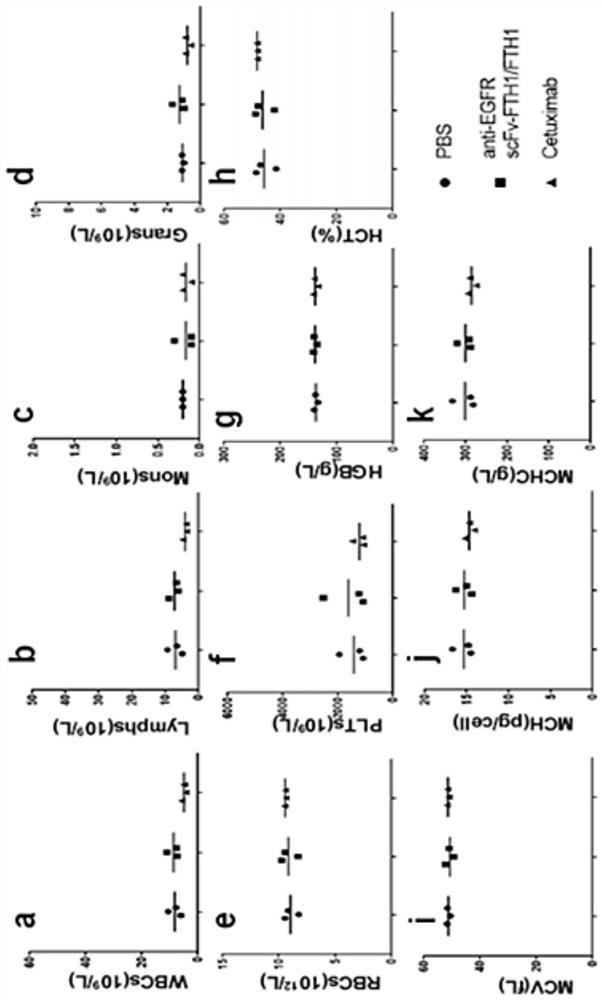
[0041] Example 2 Safety evaluation of anti-EGFR scFv::FTH1 / FTH1 protein nanoparticles in vivo
[0042] Reagent preparation:
[0043] 4% Paraformaldehyde: Dissolve 40g of paraformaldehyde in 1000mL of PBS, and filter through a 0.22μm membrane filter.
[0044] Table 1.1 Experimental grouping
[0045]
[0046]
[0047] experimental method:
[0048] As shown in Table 1.1, healthy Balb / c mice (Shanghai Experimental Animal Center, Chinese Academy of Sciences (Shanghai Slack Experimental Animal Co., Ltd.)) were randomly divided into 3 groups, 3 mice in each group, injected intraperitoneally, 1 time / 2 days , administered 3 times in a row. On the 7th day, the whole blood of the mouse was collected by picking the eyeball to contain EDTA-K 2 in the anticoagulant tube. Then measure the blood indexes of mice, the main indexes include: white blood cell number (WBCs), lymphocyte number (Lymphs), monocyte number (Mons), neutrophil number (Grans), red blood cell number (RBCs), plate...
Embodiment 3
[0077] Example 3 Anti-EGFR scFv:: Effect of FTH1 / FTH1 Protein Nanoparticles on the Survival Rate of EGFR Positive Cells
[0078] Cell line:
[0079] Human triple-negative breast cancer cell lines MDA-MB-231 and MDA-MB-468 (Shanghai Experimental Animal Center, Chinese Academy of Sciences (Shanghai Slack Experimental Animal Co., Ltd.)). Its complete medium is: L-15 medium containing 10% fetal bovine serum, culture conditions: 100% air, no CO 2 , 37 ℃ constant temperature.
[0080] Reagent preparation:
[0081] 5mg / mL MTT solution: Weigh 250mg MTT and dissolve it in 50mL PBS, dissolve it fully at 60°C, filter it with a 0.22μm sterile filter membrane, pH 7.4, and store it in the dark at -20°C.
[0082] Determination of cell viability by MTT assay
[0083] experimental method:
[0084] MDA-MB-231 and MDA-MB-468 cells grown on the adherent wall were digested with trypsin and counted, respectively in 2 × 10 3 and 5×10 3 Cells were seeded in a 96-well plate at a density of 100 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com