Isolating culture method and application of human umbilical cord mesenchymal stem cells
A technology for separating and culturing stem cells, applied in cell culture active agents, animal cells, tissue culture, etc., can solve problems such as thrombus, and achieve the effect of reducing the risk of thrombus and improving immune system diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment is a method for isolating and culturing human umbilical cord mesenchymal stem cells. The method for isolating and culturing comprises the following steps:
[0041] (a) Collect standard umbilical cord tissue, place the isolated human umbilical cord tissue in DMEM containing antibiotics (the antibiotics are penicillin and streptomycin, and the final concentration is 100U / mL), 3mM melatonin and 1% trehalose In the culture medium, store and transport at 4°C, and process the tissue within 48 hours; then wash with PBS and use surgical scissors to remove the arteries and veins, and the remaining tissue is the umbilical cord Wharton's jelly;
[0042] (b) Put the umbilical cord Wharton's jelly in a petri dish, add 10 mL of serum-free medium containing 10 μM melatonin (serum-free medium is SFM basal medium), and then cut the umbilical cord Wharton's jelly with tissue scissors Shredded jelly to 1~3mm 3 , then, place the Petri dish in 2% O 2 , 5%CO 2 Cultivate ov...
Embodiment 2
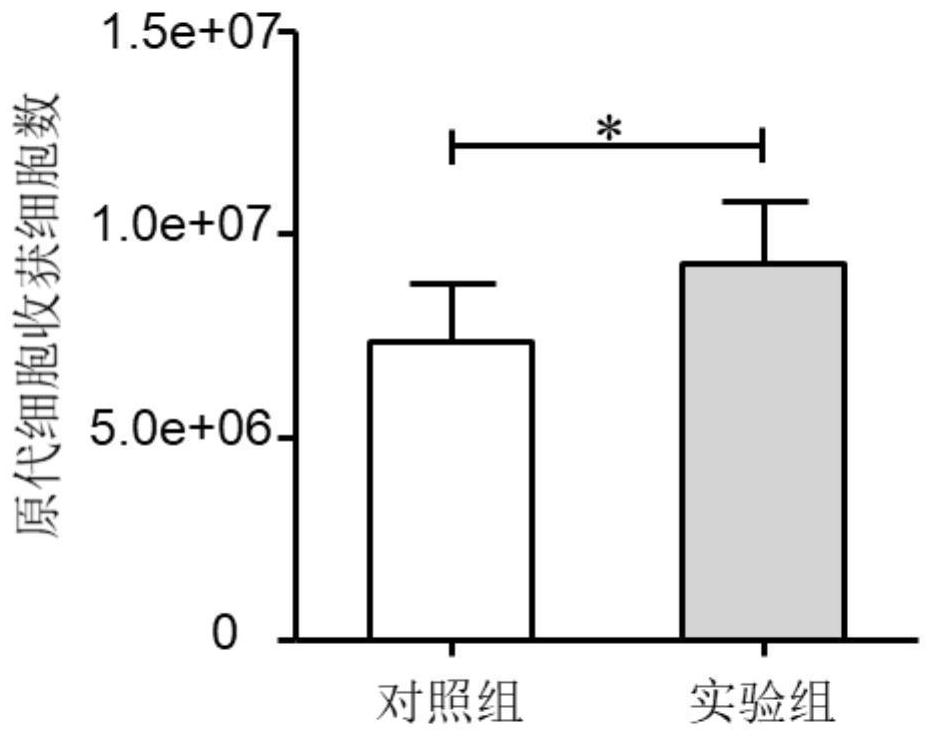
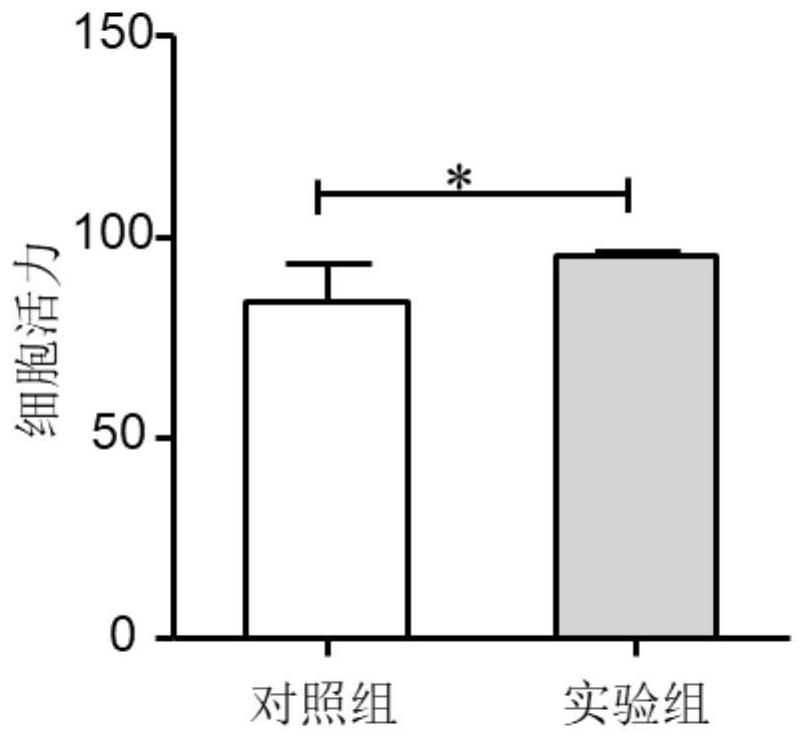
[0046] This example is a study on the number and viability of cells in the culture process of human umbilical cord mesenchymal stem cells
[0047] The research methods are:
[0048] (a) The cells harvested in Example 1 are used as the experimental group, and while the stem cells are isolated and cultured in Example 1, the non-treated control group for stem cells is set, that is, the stem cells do not add melatonin to the culture medium in the isolation and culture process, and do not use low The cells were cultured in an oxygen mode, and the rest of the operations were the same as in Example 1. After 20 days of cell culture, the cells were harvested and frozen.
[0049] (b) Rapid recovery of cryopreserved stem cells in the experimental group and control group in a 37-degree water bath.
[0050] (c) Cell number and viability detection were obtained according to the method of automatic cell counter.
[0051] During the whole process of umbilical cord mesenchymal stem cell iso...
Embodiment 3
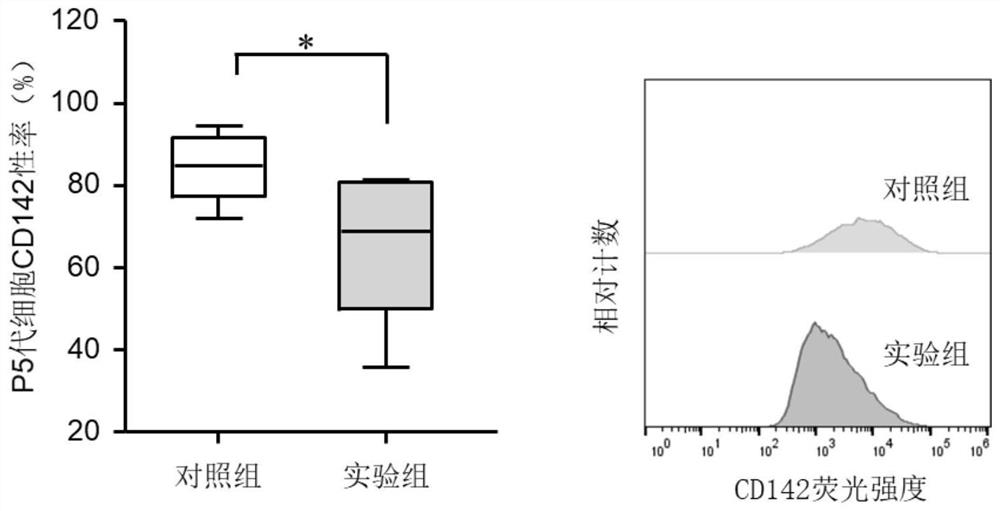
[0054] This example is flow cytometry detection of tissue factor and chemokine expression of human umbilical cord mesenchymal stem cells obtained by different culture methods:
[0055] (1) According to Table 1, take 1.5*10^6 cells and centrifuge at 1500rpm for 7min. Discard the supernatant, add 4mL PBS to resuspend the cells, and centrifuge at 1500rpm for 7min. Then add 1.5mL PBS to resuspend the cells, and use nylon mesh to filter the cell suspension.
[0056] (2) Take 4 flow-type test tubes, mark 1 to 4, and add the flow-type antibody combination in advance (use a pipette to add antibodies to different positions at the bottom of the flow-type test tubes to prevent flow-type antibodies from crossing).
[0057] (3) Add 100 μL of cells (at least 1.0*10^5 cells) to each test tube on the machine, filter the cells with a 300 mesh (40 μM) cell sieve, fully vibrate and mix the antibody and cell suspension, and centrifuge instantaneously to sediment the cells on the tube wall to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com