Medical application of aporphine alkaloid
A technology of alkaloids and uses, applied in the field of pharmacotherapeutics, can solve problems such as low response rate and slow onset time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
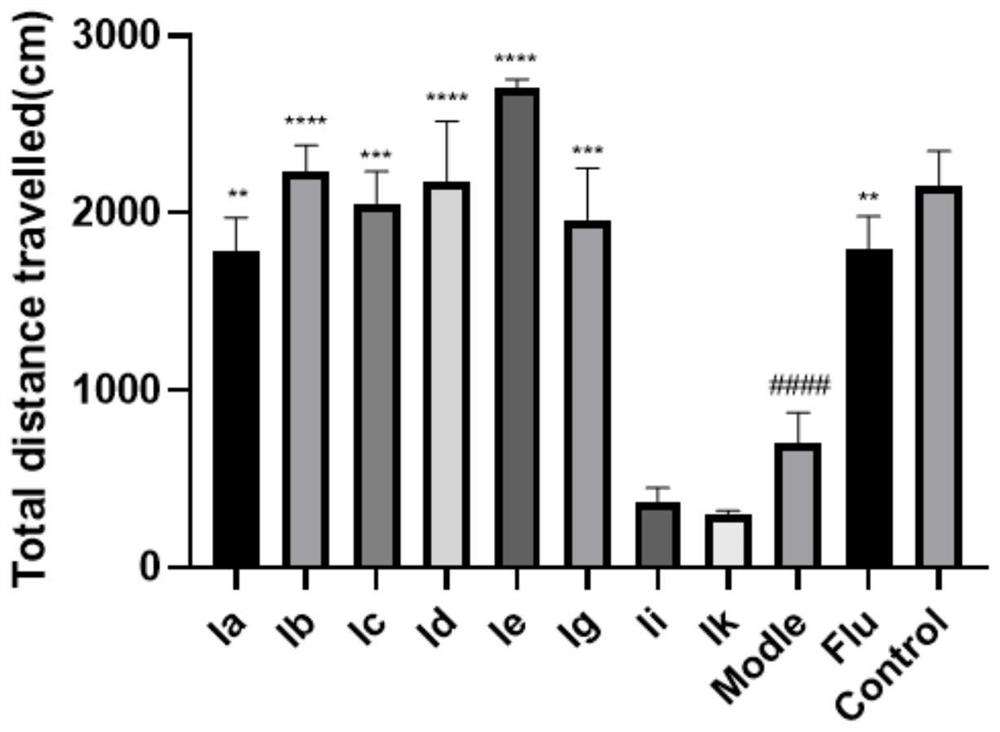
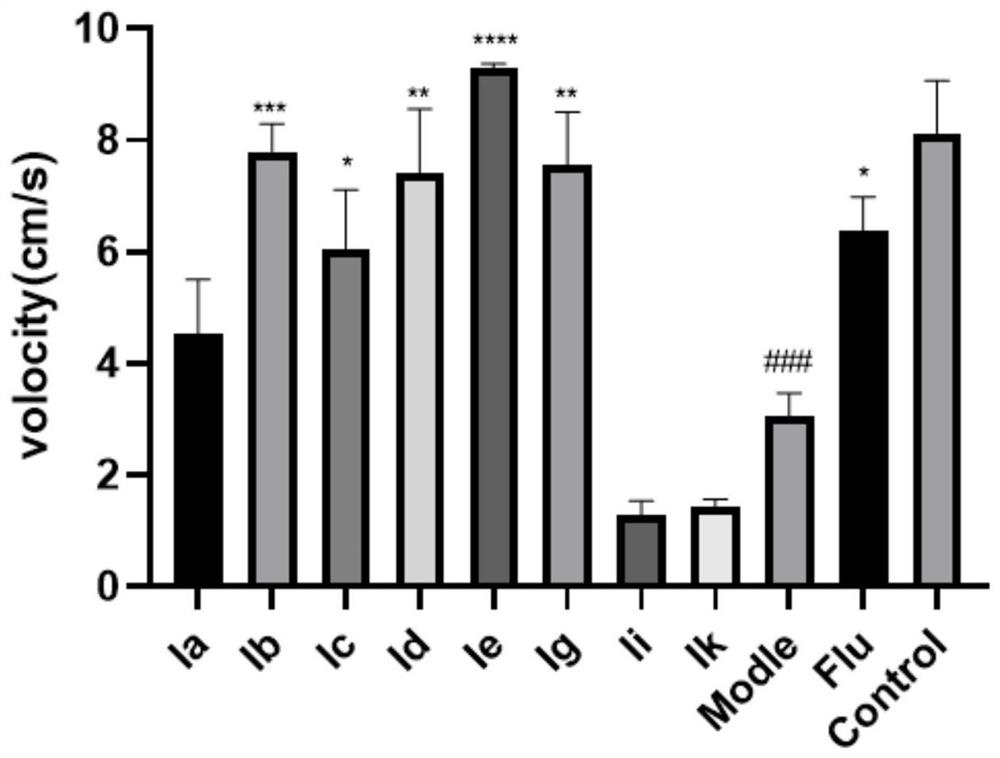
[0037] Embodiment 1: Zebrafish in vivo pharmacological experiment of the first series of aporphi alkaloids
[0038] The open field test (OFT) was used to test the antidepressant activity of the reserpine zebrafish depression model, and fluoxetine (Flu) was selected as the positive control drug.
[0039] Instrument: Zebrafish Behavior Analyzer
[0040] Reagents: compounds Ia, Ib, Ic, Id, Ie, Ig, Ii, Ik (all known compounds); fluoxetine, reserpine (purity ≥ 99.0%), purchased from Sigma Aldrich (Shanghai) Trading Ltd.
[0041] Zebrafish: wild-type AB adult zebrafish, provided by Wuhan National Zebrafish Resource Center, and bred in the zebrafish laboratory of Nanjing Ruiying Runze Biomedical Technology Co., Ltd. The zebrafish breeding method is carried out according to The ZebrafishBook, and it is cultured in circulating water , the water quality is maintained through the circulating water system, the water temperature is kept at 28.5°C, the daily fixed light and dark time is 1...
Embodiment 2
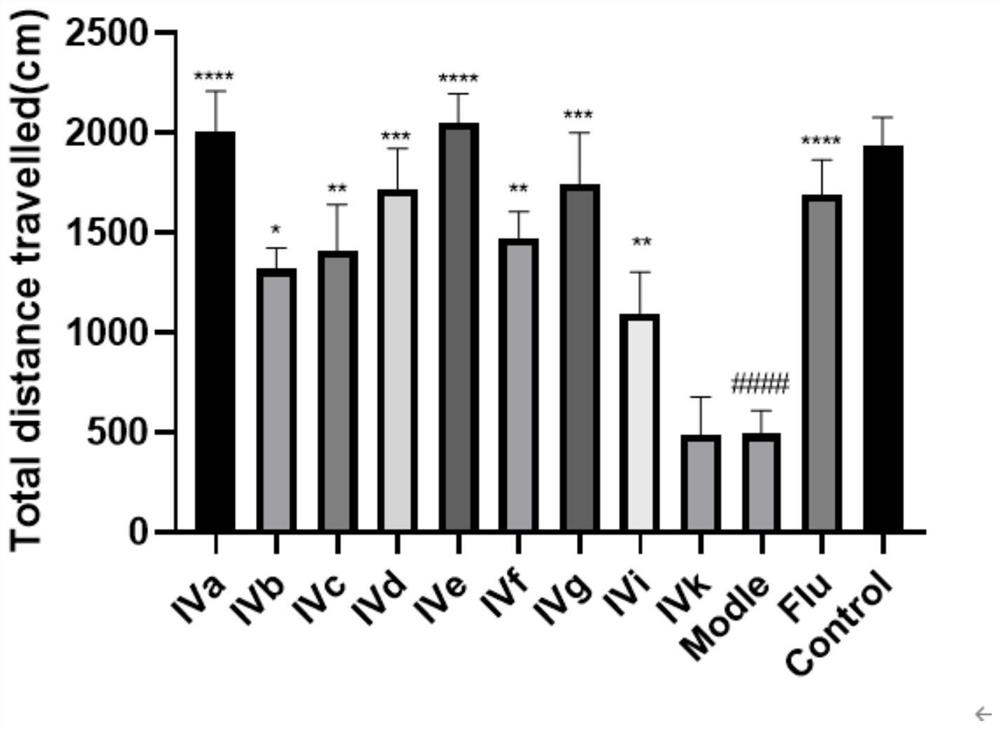
[0044] Embodiment 2: Zebrafish in vivo pharmacological experiments of IV series of aporphi alkaloids
[0045] Reagents: Compounds IVa, IVb, IVc, IVd, IVe, IVf, IVg, IVi, IVk (all known compounds).
[0046] Zebrafish were randomly divided into 12 groups, blank control group (10), model group (10), positive control group (10, fluoxetine 1 μ M), administration group: IVa group (10, 1 μ M), Group IVb (10 strips, 1 μM), Group IVc (10 strips, 1 μM), Group IVd (10 strips, 1 μM), Group IVe (10 strips, 1 μM), Group IVf (10 strips, 1 μM), Group IVg (10 strips , 1 μM), IVi group (10 pieces, 1 μM), IVk group (10 pieces, 1 μM), and the rest are the same as in Example 1.
[0047] Result: if image 3 and Figure 4 As shown, compared with the blank control group, the total moving distance and swimming speed of the model group zebrafish were significantly reduced ( #### P**** P*** P** P* P<0.05).
Embodiment 3
[0048] Embodiment 3: the zebrafish in vivo pharmacological experiment of Ie
[0049]
[0050] Zebrafish were randomly divided into 4 groups, blank control group (10), model group (10), positive control group (10, fluoxetine 1 μM), Ie group (10, 1 μM). The animal trajectory tracking system traces the following variables of the zebrafish movement curve within 5 minutes: A total swimming distance; B average speed; C immobility time; D immobility frequency; E meandering degree; F rotation angle; G angular velocity. All the other are with embodiment 1.
[0051] Result: if Figure 5 and Image 6 As shown, compared with the blank group, the total moving distance and swimming speed of the model group zebrafish were significantly reduced ( #### P#### P**** P**** P<0.001), and all the behavioral indicators showed that compound Ie had the best effect, showing better antidepressant efficacy than fluoxetine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com