A kind of wet oxidation catalyst and its preparation method and application
A wet oxidation and catalyst technology, used in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc. The effect of improving catalytic activity, reducing production cost and reducing operating temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The present invention provides a method for preparing a wet oxidation catalyst described in the above scheme, comprising the following steps: mixing a divalent nickel salt, a ferric salt, a precipitating agent and water to obtain a mixed solution;
[0032] adjusting the pH value of the mixed solution to 8-10, performing a hydrothermal reaction on the mixed solution after the adjusted pH value, and sequentially drying and calcining the solid product obtained by the hydrothermal reaction to obtain a nickel-iron hydrotalcite carrier;
[0033] Mixing the nickel-iron hydrotalcite carrier, the Pt precursor and water to obtain a mixed material liquid; the mass of Pt in the Pt precursor is 0.001% to 1% of the mass of the nickel-iron hydrotalcite carrier;
[0034] Mixing the mixture liquid with a reducing agent aqueous solution to carry out a reduction reaction, separating solids and liquids from the reduction reaction product, drying the solid product, and roasting the obtained ...
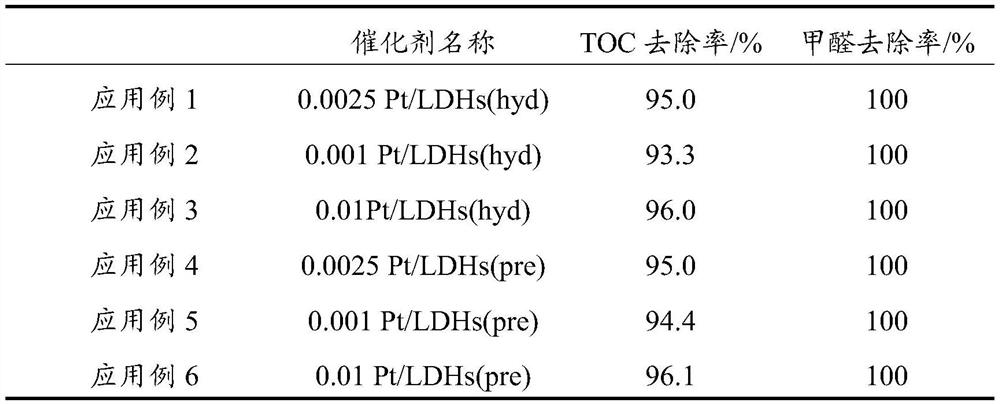
Embodiment 1
[0070] (1) 2.44gNi(NO 3 ) 2 ·6H 2 O, 1.13gFe(NO 3 ) 3 9H 2 O was dissolved in 30 mL deionized water;
[0071] (2) 1.26g of urea was dissolved in 30mL of deionized water, mixed with the solution in (1), and stirred evenly to obtain a mixed solution;
[0072] (3) adding dropwise sodium hydroxide solution to the above mixed solution to adjust the pH value of the solution to 8.5;
[0073] (4) The mixed solution after adjusting the pH value is placed in a hydrothermal reaction kettle, the reaction temperature is 50°C, and the reaction time is 19h;
[0074] (5) After hydroheating, the obtained product is suction filtered, washed, dried, and placed in a muffle furnace for calcination at 400° C. for 6 hours to obtain a nickel-iron hydrotalcite carrier, which is denoted as LDHs (hyd);
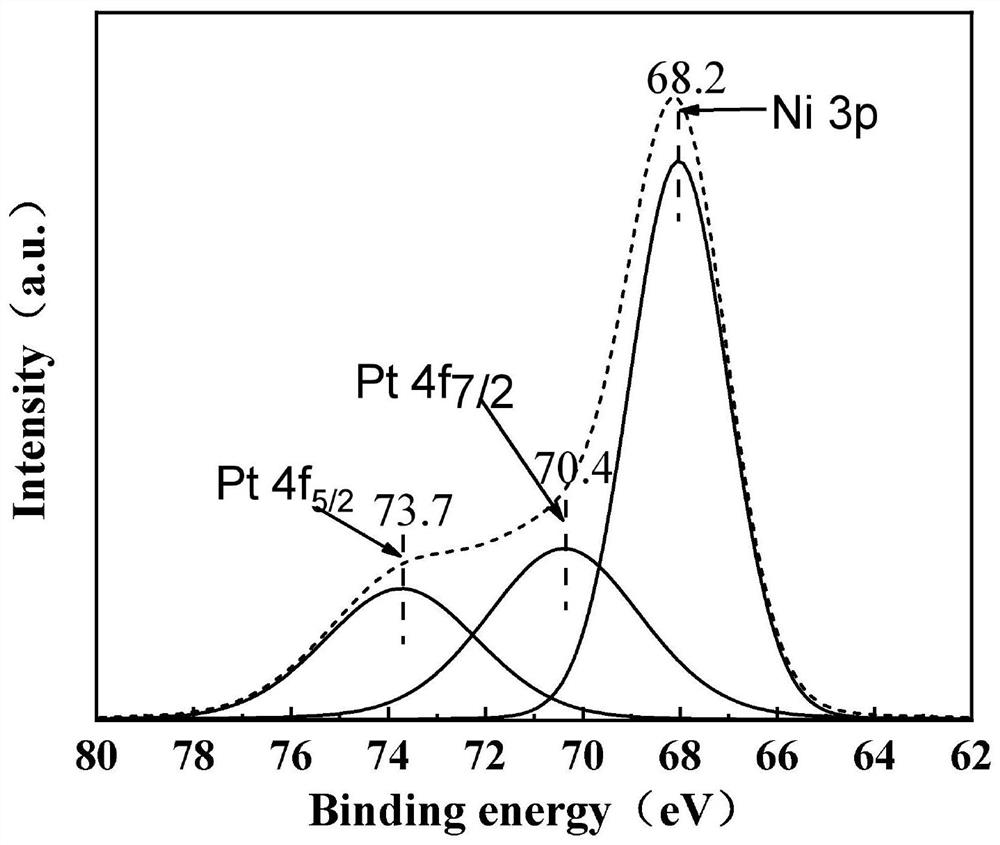
[0075] (6) Disperse 1 g of LDHs (hyd) in 75 mL of water, add 6.65 μL of chloroplatinic acid hexahydrate solution under stirring conditions, and soak for 30 minutes, wherein the concentration of c...
Embodiment 2
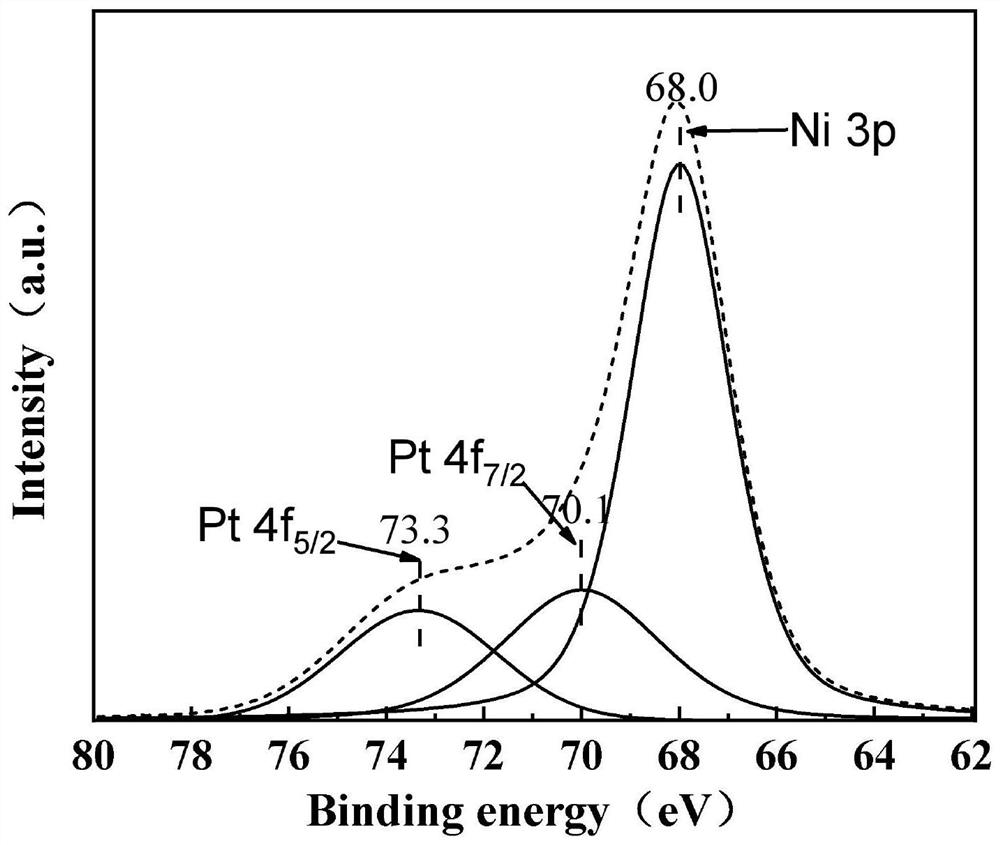
[0080] The only difference from Example 1 is that the addition amount of chloroplatinic acid hexahydrate solution is adjusted to 2.66 μL, and the obtained catalyst is recorded as 0.001Pt / LDHs(hyd).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com