Safe synthesis method of 1,3,5-triamino-2,4,6-trinitrobenzene
A technology of triaminobenzene and trinitrobenzene, applied in the field of safe synthesis of 1,3,5-triamino-2,4,6-trinitrobenzene, which can solve the impact of chlorine-containing by-products and harsh reaction conditions , cost reduction and other issues, to achieve the effect of avoiding separation, purification and collection, simplifying the operation process and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
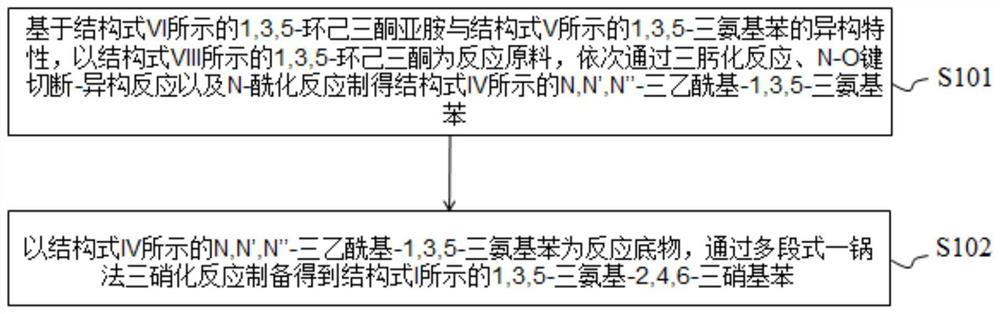
[0035] The embodiment of the present invention provides a safe synthesis method of 1,3,5-triamino-2,4,6-trinitrobenzene shown in structural formula I, refer to figure 1 Shown, this synthesis method comprises:
[0036] Step 1 (S101), based on the isomerism characteristics of 1,3,5-cyclohexanetriketone imine shown in structural formula VI and 1,3,5-triaminobenzene shown in structural formula V, with structural formula VIII 1,3,5-Cyclohexanetrione is used as the reaction raw material, and the N,N',N"-three Acetyl-1,3,5-triaminobenzene.
[0037] In this implementation step, first, 1,3,5-cyclohexanone oxime reacts with hydroxylamine under alkaline conditions to obtain 1,3,5-tricyclohexanone oxime represented by structural formula VII in high yield; Then, the 1,3,5-tricyclohexanone oxime shown in the structural formula VII cuts the N-O bond under the catalysis of Raney nickel and other catalysts to obtain the 1,3,5-triaminobenzene shown in the structural formula V; finally, the st...
Embodiment 1
[0063] Step 1: Preparation of N,N,N-triacetyl-1,3,5-triaminobenzene represented by structural formula IV.
[0064] Take hydroxylamine hydrochloride (500.4g, 7.2mol, 6eq) and dissolve it in concentrated ammonia water (25%, 540ml, 7.2mol, 6eq), add 2250ml of water to make it fully dissolved, then weigh 1,3,5-cyclohexanetrione ( 194.4g, 1.2mol, 1eq), the reaction was continued under vigorous stirring at 5°C for 5 hours, and the suspension was filtered, washed and dried to obtain powder solid 1,3,5-cyclohexanetrione oxime (192.7g, yield 94 %).
[0065] It should be noted that when hydroxylamine hydrochloride is fully dissolved and 1,3,5-cyclohexanetrione is directly added, the degree of dissolution of 1,3,5-cyclohexanetrione is low, resulting in low reaction efficiency and difficult identification of the reaction End point and low yield, etc., therefore, in order to solve these problems, in this implementation step, after hydroxylamine hydrochloride is fully dissolved, deionized ...
Embodiment 2
[0090] Step 1: Preparation of N,N,N-triacetyl-1,3,5-triaminobenzene represented by structural formula IV.
[0091] Dissolve hydroxylamine hydrochloride (500.4g, 7.2mol, 6eq) in concentrated ammonia water (25%, 1620ml, 21.6mol, 18eq), add 1000ml of water to fully dissolve it, and weigh 1,3,5-cyclohexanetrione Glycinol (194.4g, 1.2mol, 1eq), under ice bath (-5°C), continued to react with vigorous stirring for 5 hours, the obtained suspension was suction filtered, washed and dried to obtain powder solid 1,3,5-tris Cyclohexanone oxime (135.4 g, 66% yield).
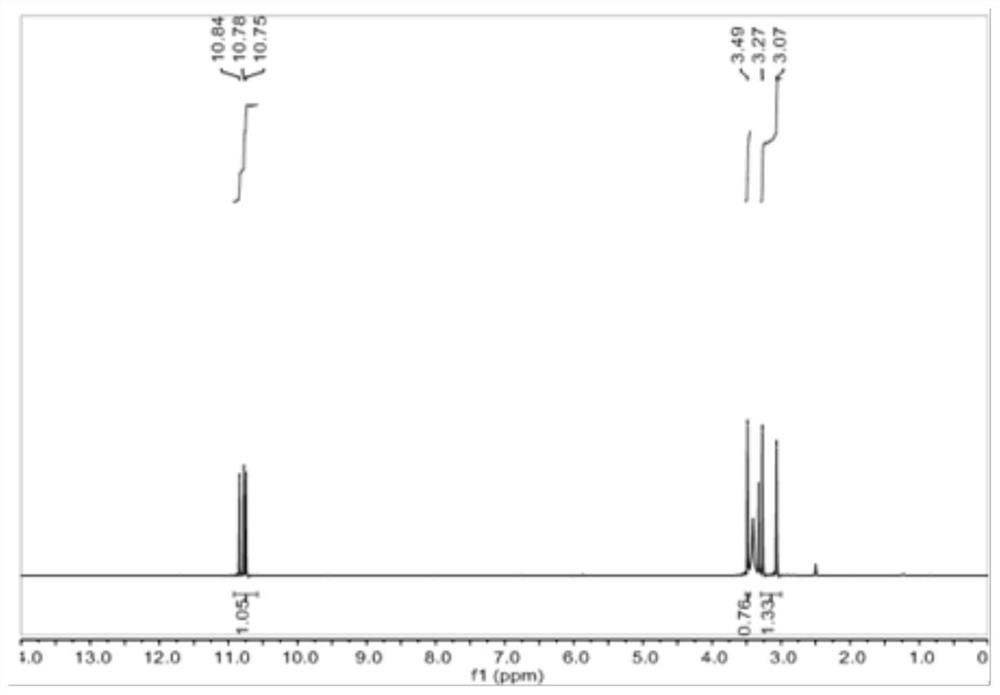
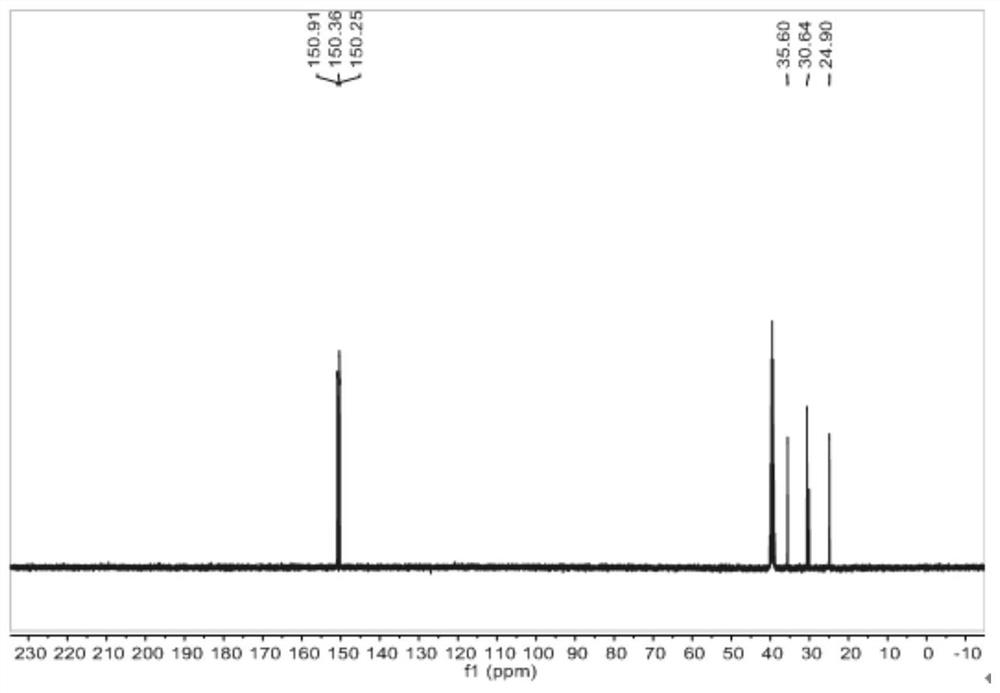
[0092] The proton nuclear magnetic resonance spectrum of the 1,3,5-tricyclohexanone oxime shown in the structural formula VII of the embodiment of the present invention 2, the carbon nuclear magnetic resonance spectrum and the infrared spectrum are respectively with figure 2 , image 3 and Figure 4Same, not repeated.
[0093] Place the prepared 1,3,5-tricyclohexanone oxime (200g, 1.2mol, 1eq) and the Raney nickel catalys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com