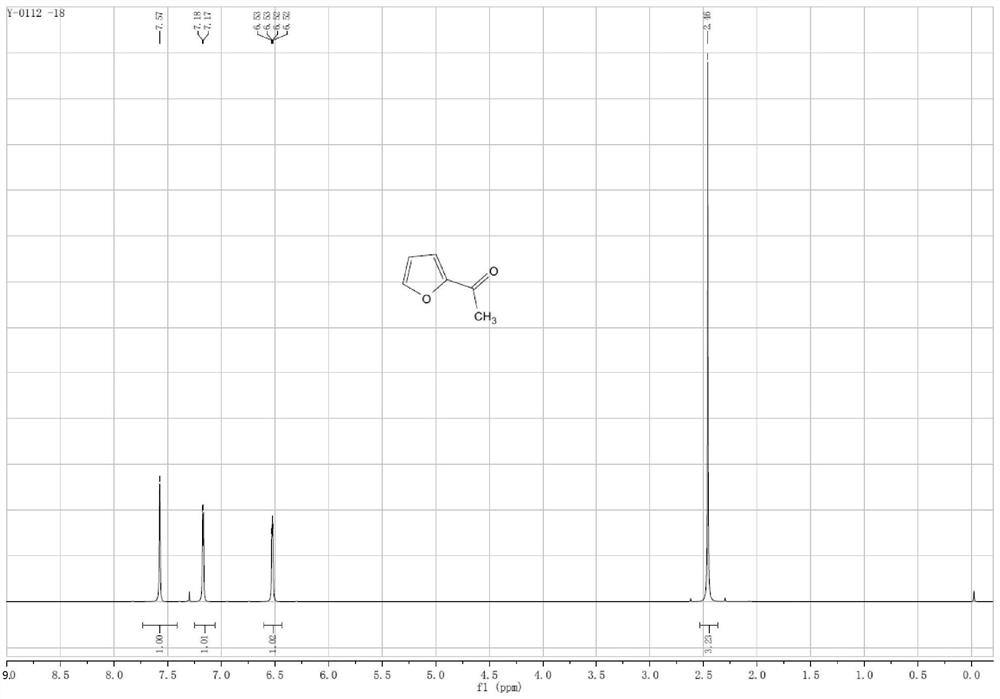
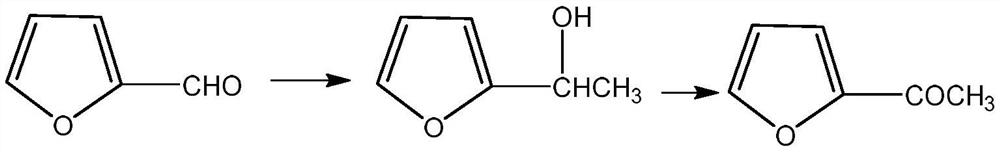
Preparation method of 2-acetylfuran
A technology for acetylfuran and furanmethyl alcohol, which is applied in the field of preparation of 2-acetylfuran, can solve the problems of restricting product market stability, poor atomic economic efficiency, and high safety risks, and achieves low production cost, satisfactory stability, and high catalyst performance. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0034] The preparation method of embodiment 12-acetylfuran
[0035] 1. Format response:
[0036] Add 100 ml of tetrahydrofuran solution of 3 moles per liter of methylmagnesium chloride to the reactor, cool down to about zero, slowly add 25 ml of furfural, keep the internal temperature not exceeding 25 ° C, and slowly drop the reaction solution after the reaction is completed. Add it to 100 ml of ice water, control the internal temperature not to exceed 25°C, then slowly add dilute hydrochloric acid dropwise to adjust the pH of the reaction solution to about 7, separate the organic layer, and then concentrate to dryness to obtain furan methyl alcohol. 99%.
[0037] 2. Oxidation reaction:
[0038] Add 100 milliliters of ethylene dichloride, 27.5 grams of furan methyl alcohol, 0.8 gram of 4-hydroxyl-2,2,6,6-tetramethylpyridine oxide (Temp), 0.6 gram of potassium bromide in the reactor, Control the temperature at 25° C. and feed air or oxygen to react for 3 to 5 hours. After th...
Embodiment 2-5
[0041] The difference from Example 1 is that the solvent used in the oxidation reaction is different: dichloromethane, trichloromethane, tetrahydrofuran or acetonitrile are used instead of dichloroethane.
Embodiment 6-10
[0043] The difference from Example 1 is that the temperature of the oxidation reaction is different: the temperatures are 0, 10°C, 20°C, 30°C, and 40°C, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com