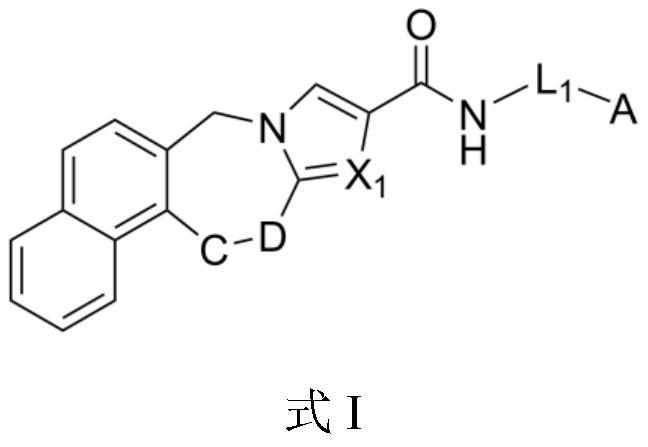
Tetracyclic compound as plasma kallikrein inhibitor and application thereof
A compound and solvate technology, applied in the field of tetracyclic compounds, can solve the problems of poor physical and chemical properties of compounds, poor oral bioavailability, poor selectivity of related enzymes serine protease, etc., and achieves high selectivity, novel structure and good activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
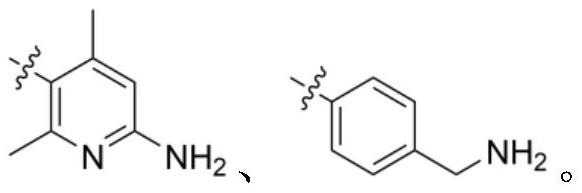
[0074] N-((6-amino-2,4-dimethylpyridin-3-yl)methyl)-12,13-dihydro-7H-imidazol[1,2-a]naphthalene[1,2-e] [1,4]diazepine - Preparation of 10-carboxamide
[0075]
[0076] Step a): Preparation of ethyl 2-amino-2-(hydroxyimino)acetate
[0077] Water (110 mL) was slowly added to ethyl cyanoformate (30.0 g, 0.303 mol), hydroxylamine hydrochloride (31.6 g, 0.455 mol) and sodium carbonate (80.3 g, 0.758 mol) in ethanol (200 mL) mixture, and the addition was completed , the reaction solution was stirred at 20°C for 10 h. After the reaction, the solvent was evaporated under reduced pressure, the residue was extracted with ethyl acetate (200 mL×3), the organic layers were combined, dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness under reduced pressure to obtain 2-Amino-2-(hydroxyimino) ethyl acetate, yield 65.0%, 1 H NMR (400MHz, DMSO-d 6 )δ10.66-9.12(m,1H),5.79-5.31(m,2H),4.07-3.97(m,2H),1.18-1.13(m,3H); ESI-MS(m / z):133.2[ M+H] + .
[...
Embodiment 2
[0094] N-(4-(aminomethyl)benzyl)-12,13-dihydro-7H-imidazo[1,2-a]naphthalene[1,2-e][1,4]diazepine - Preparation of 10-carboxamide
[0095]
[0096] Step a): (4-((12,13-Dihydro-7H-imidazol[1,2-a]naphthalene[1,2-e][1,4]diazepine Preparation of -10-carboxamide)methyl)benzyl)carbamate tert-butyl ester
[0097] 12,13-dihydro-7H-imidazol[1,2-a]naphthalene[1,2-e][1,4]diazepine -10-Formic acid (100mg, 0.358mmol), (4-(aminomethyl)benzyl) tert-butyl carbamate (127mg, 0.537mmol), HBTU (204mg, 0.537mmol), triethylamine (80mg, 0.573mmol ) and DMF (3mL) were added to the reaction flask, and stirred at room temperature for 5h. After the reaction, the reaction solution was diluted with water, extracted with ethyl acetate (20mL×3), and the organic layers were combined, followed by water (10mL×2) and saturated chlorine Wash with sodium chloride aqueous solution (10mL×2), dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain a light yell...
Embodiment 3
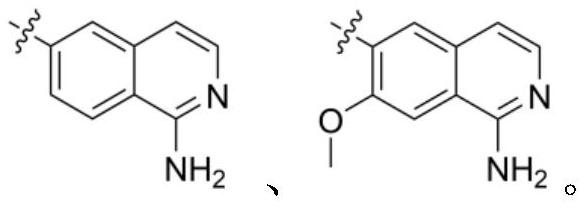
[0101] N-((1-aminoisoquinolin-6-yl)methyl)-12,13-dihydro-7H-imidazol[1,2-a]naphthalene[1,2-e][1,4]di Aza - Preparation of 10-carboxamide
[0102]
[0103] The operation process is the same as in Example 1, except that 5-(aminomethyl)-4,6-dimethylpyridine-2-diamine dihydrochloride in step i is treated with 6-(aminomethyl)isoquinoline- 1-amine substitution, N-((1-aminoisoquinolin-6-yl)methyl)-12,13-dihydro-7H-imidazol[1,2-a]naphthalene[1,2-e] [1,4]diazepine -10-Formamide; ESI-MS(m / z): 435.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com