Modified luciferase mutant protein, bioluminescent probe, probe group, preparation method and detection method
A luciferase and bioluminescence technology, applied in the field of molecular biology, can solve the problems of inability to real-time and accurate response, limited application of nucleic acid markers, restriction of bioluminescence application, etc., to achieve quantitative detection, high signal-to-noise ratio, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The present invention provides a method for preparing the modified luciferase mutant protein described in the above technical scheme, comprising the following steps: mutating the cysteine at the 166th position of luciferase to serine, and Addition of a linking region containing a cysteine site to the C-terminus of luciferase results in DNA encoding an engineered luciferase mutant protein whose The nucleotide sequence is shown in SEQ ID NO.2; the DNA encoding the transformed luciferase mutant protein is inserted into a plasmid vector to obtain a recombinant plasmid; the obtained recombinant plasmid is transformed into Escherichia coli to obtain a recombinant large intestine Bacillus; culturing the recombinant Escherichia coli to obtain the modified luciferase mutant protein.
[0024] In the present invention, the cysteine at the 166th position of luciferase is mutated into serine, and a linking region containing cysteine site is added to the C-terminus of lucifer...
Embodiment 1
[0035] Preparation of modified luciferase mutant protein
[0036] (1) Construction of the transformed luciferase mutant expression vector: respectively amplify the N-terminal and C-terminal coding sequences of luciferase by PCR, and insert cysteine at the luciferase 166 site Acid codons were mutated to serine codons, and a Linker coding sequence containing cysteine was added after the C-terminal coding sequence; PCR-amplified fragments were recovered by gel electrophoresis and luciferase was amplified by overlap PCR The complete coding sequence of the mutant, and NdeI and HindIII restriction sites were added at the 5' and 3' ends respectively; after the above fragment was recovered by electrophoresis and digested, it was combined with the pET-25(b+) vector digested with NdeI and HindIII connected, and the correctness of the clone was verified by sequencing; the primers used in PCR are shown in SEQ ID NO.6-SEQ ID NO.8.
[0037] (2) Expression and purification of the modifi...
Embodiment 2
[0039] Construction of Bioluminescent Probes for Detecting Nucleic Acid Molecules
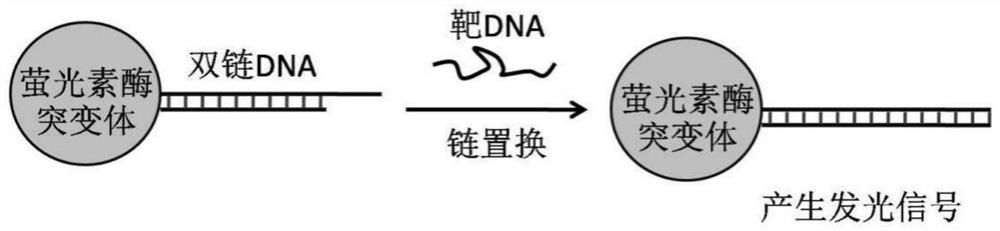
[0040] The structure of the bioluminescent probe for detecting nucleic acid molecules provided by the invention is as follows: figure 1 As shown, its construction method is as follows:
[0041] (1) Coupling of double-stranded DNA:
[0042] A) Functionalization of DNA by SMCC: Incubate 1 mM first-strand single-stranded DNA with amino modification at the 5' end and 1.2 mM second-strand single-stranded DNA with Dabcyl group modification at the 3' end in PBS solution for 1 h , wherein the nucleotide sequence of the first strand is as shown in SEQ ID NO.2, and the nucleotide sequence of the second strand is as shown in SEQ ID NO.3; adding the SMCC that the final concentration is 20mM in the above solution, reverse mixing After homogenization, incubate at 25°C for 2 hours; further add 1 / 10 volume of 3M NaAc (pH=5.3), and add 1 volume of isopropanol, incubate at room temperature for 30 minutes; cent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com