Preparation method and application of carbon-loaded nickel catalyst
A catalyst and nickel-supported technology, which can be used in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc. The effect of high selectivity of hydrogen peroxide, convenient continuous production and simple process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Catalyst preparation
[0030] a. Precipitation deposition method: In the ethylene glycol solution containing metal organic framework structure (ZIF-8), add a certain concentration of sodium hydroxide aqueous solution, and then mix it with the ethylene glycol solution containing nickel nitrate, hydrothermal treatment, in ZIF A certain amount of nickel salt is deposited on the surface of -8.
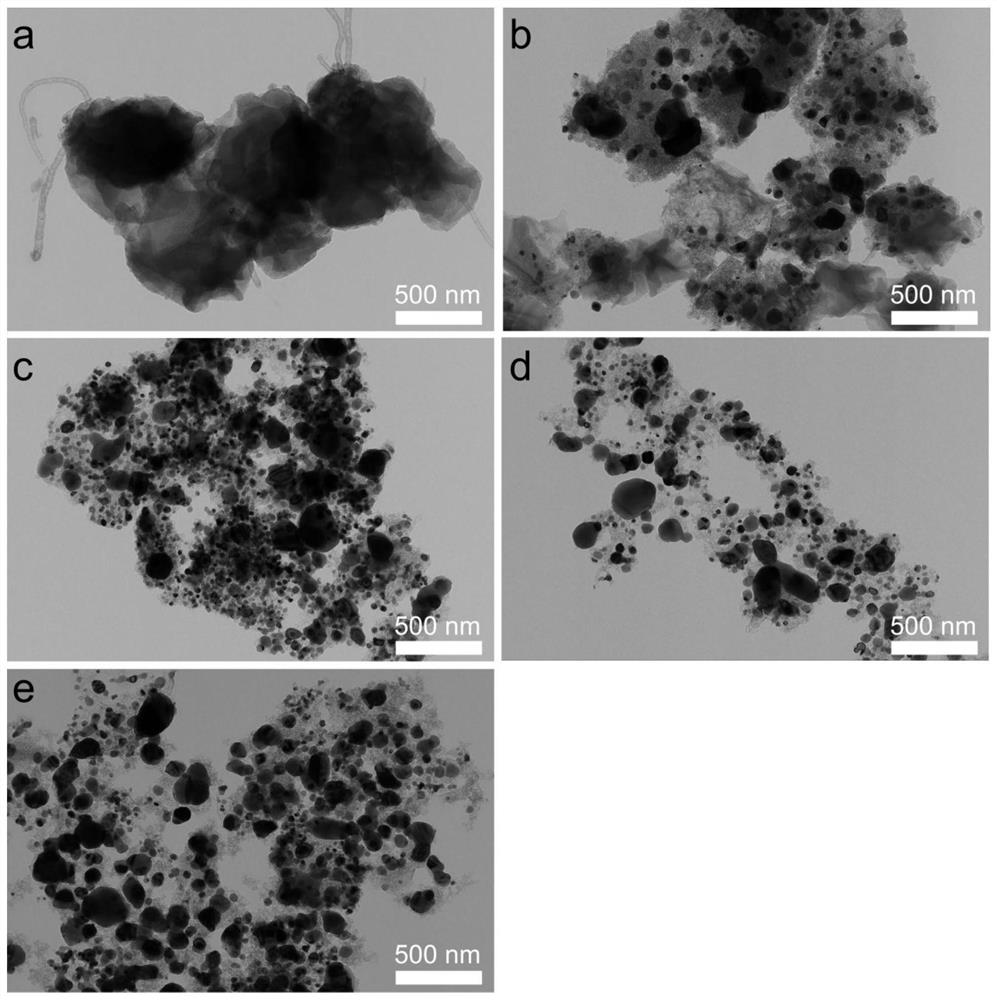
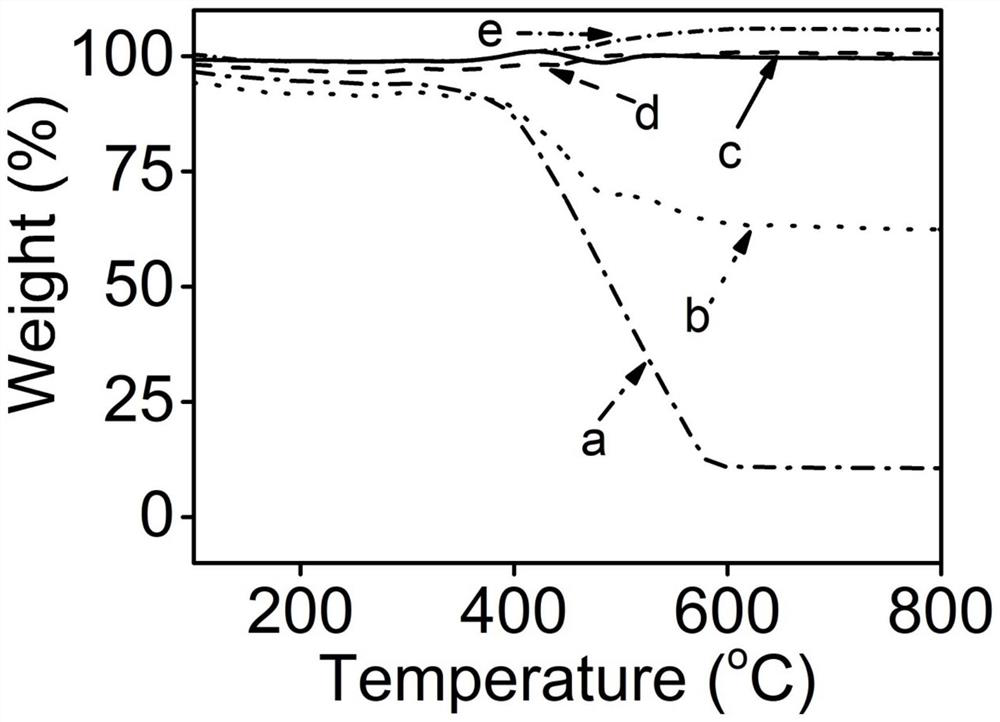
[0031] b. After calcining the metal nickel salt deposited in step a at 900°C for 2 h in an argon atmosphere, a carbon-supported nickel catalyst was obtained (Ni / C-X, X represents the mass ratio of nickel nitrate to ZIF-8). Transmission electron microscopy results of the resulting product can be found in figure 1 , see the thermogravimetric spectrum figure 2 ;
[0032] Electrochemical performance test
[0033] Weigh 8 mg of the catalyst powder obtained by the method of the present invention, disperse it in 1000 uL of isopropanol aqueous solution containing 0.1% naphthol solution...
Embodiment 2
[0039] Catalyst preparation
[0040] a. Precipitation deposition method: In the ethylene glycol solution containing carbon black (XC-72), add a certain concentration of sodium hydroxide aqueous solution, then mix with the ethylene glycol solution containing nickel nitrate, hydrothermal treatment, on the surface of carbon black A certain amount of nickel salt was deposited by precipitation, and the mass ratio of nickel nitrate to carbon black was 8.
[0041] b. After calcining the metal nickel salt deposited in step a at 900°C for 2 h in an argon atmosphere, the carbon-supported nickel catalyst (Ni / XC-72) was obtained.
[0042] Electrochemical performance test
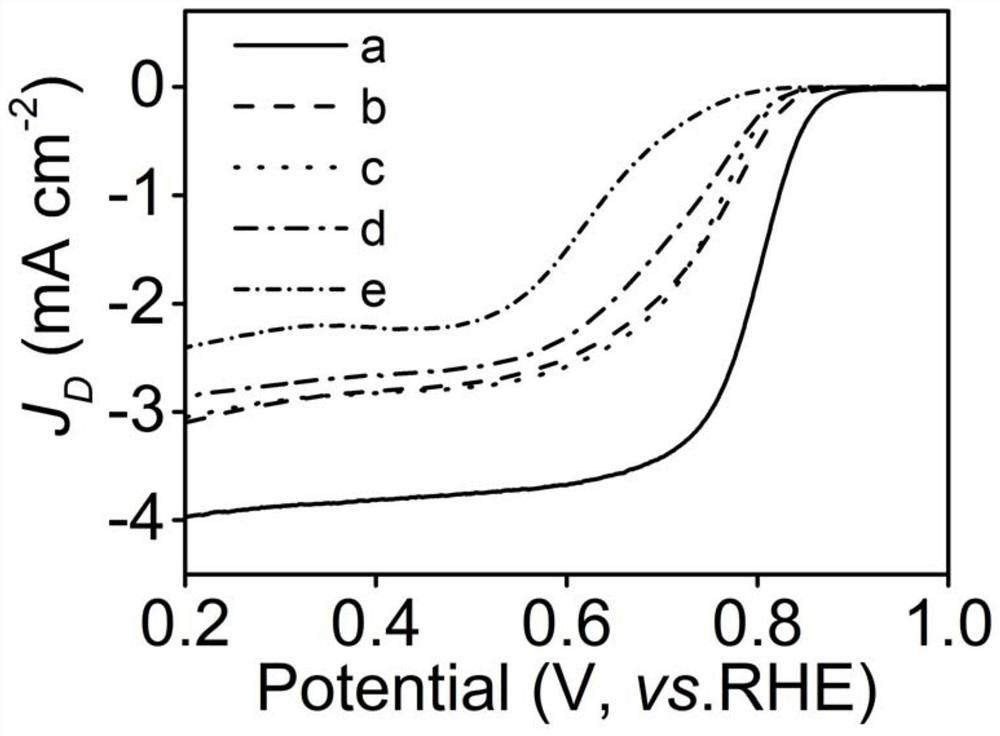
[0043] The test method is the same as in Example 1, except that the disc electrode of the ring-disk electrode is modified with Ni / XC-72, and the specific rotating ring-disk electrode is used to test the selectivity of hydrogen peroxide, see Figure 7 .
Embodiment 3
[0045] Catalyst preparation
[0046] a. Precipitation deposition method: In the ethylene glycol solution containing carbon black (XC-72), add a certain concentration of sodium hydroxide aqueous solution, then mix with the ethylene glycol solution containing nickel chloride, hydrothermal treatment, in the carbon black A certain amount of nickel salt was deposited on the surface, and the mass ratio of nickel chloride to carbon black was 8.
[0047] b. After calcining the metal nickel salt deposited in step a at 900°C for 2 h in an argon atmosphere, the carbon-supported nickel catalyst (Ni-2 / XC-72) was obtained.
[0048] Electrochemical performance test
[0049] The test method is the same as in Example 1, except that the disc electrode of the ring-disk electrode is modified with Ni-2 / XC-72, and the specific rotating ring-disk electrode is used to test the selectivity of hydrogen peroxide, see Figure 7 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com