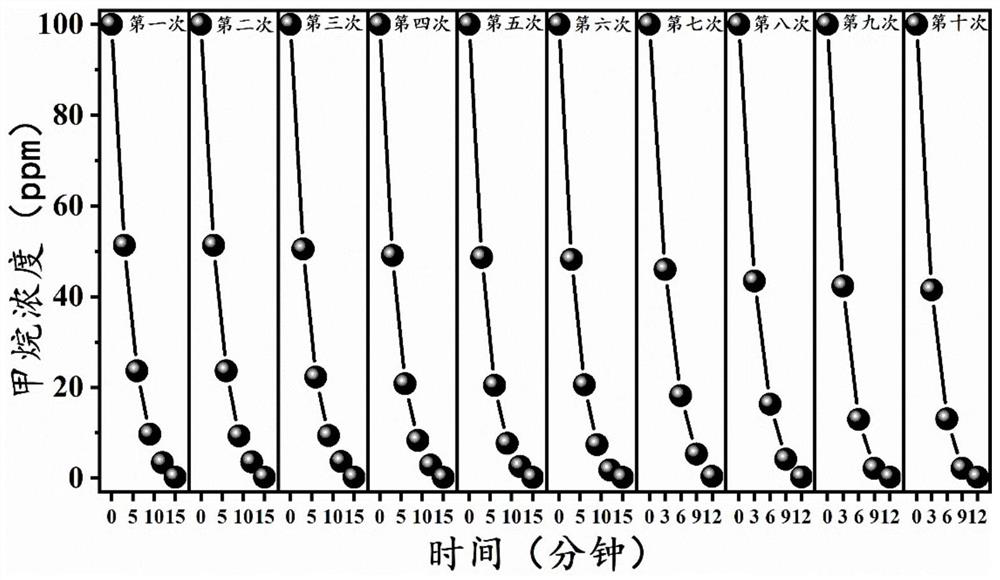
Mn-ZnO catalyst as well as preparation method and application thereof
A catalyst and mass ratio technology, applied in chemical instruments and methods, physical/chemical process catalysts, separation methods, etc., can solve problems such as reduced catalytic performance, limited applications, and strong recombination capabilities of photogenerated electrons and holes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The present invention provides the preparation method of the Mn-ZnO catalyst described in the above technical scheme, comprising the following steps:
[0027] (1) mixing zinc salt, manganese salt and water to obtain a mixed solution;
[0028] (2) mixing the mixed solution obtained in the step (1) with oxalic acid and carrying out a precipitation reaction to obtain a mixture of zinc oxalate and manganese oxalate;
[0029] (3) Annealing the mixture of zinc oxalate and manganese oxalate obtained in the step (2) to obtain a Mn-ZnO catalyst.
[0030] Unless otherwise specified, the present invention has no special limitation on the source of each component, and commercially available products well known to those skilled in the art can be used.
[0031] The invention mixes zinc salt, manganese salt and water to obtain a mixed solution.
[0032] In the present invention, the zinc salt is preferably at least one of zinc nitrate and zinc acetate, more preferably zinc acetate, ...
Embodiment 1
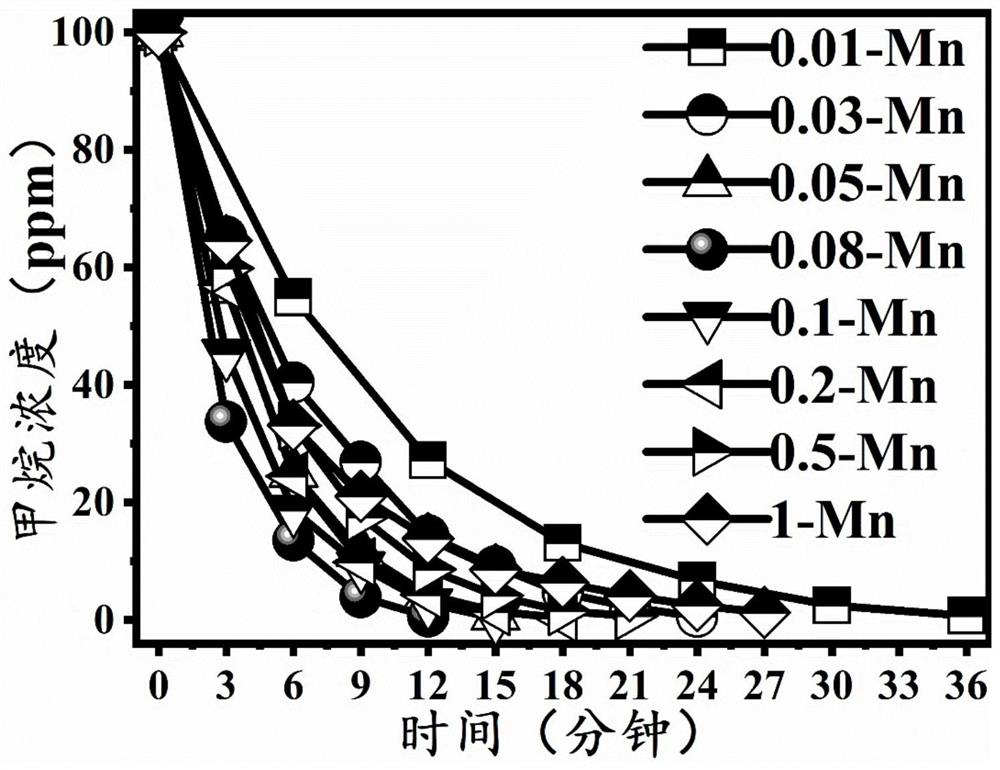
[0057] 0.01-Mn-ZnO catalyst: composed of ZnO and Mn doped in the ZnO, the mass ratio of Mn and ZnO is 0.01:100;
[0058] Preparation:
[0059] (1) Mix deionized water and manganese acetate tetrahydrate to obtain 0.0816mol / L manganese acetate solution, take 22.3 μL manganese acetate solution and add 2.6967g zinc acetate dihydrate (can prepare 1g ZnO) and then add 50mL deionized water, Stir for 15min at 25°C to obtain a mixed solution (the ratio of the amount of manganese acetate tetrahydrate and zinc acetate dihydrate is 0.015:100; the volume ratio of the total mass of manganese acetate tetrahydrate and zinc acetate dihydrate to deionized water is 2.7 g: 50mL; the mass of Mn in the mixed solution and the mass ratio of ZnO are 0.01:100);
[0060] (2) Under stirring conditions at 25°C, add 5.40 g of oxalic acid dihydrate to the mixed solution (the mass ratio of the total mass of manganese acetate tetrahydrate and zinc acetate dihydrate to oxalic acid dihydrate is 1:2), stir for ...
Embodiment 2
[0063] 0.03-Mn-ZnO catalyst: composed of ZnO and Mn doped in the ZnO, the mass ratio of Mn and ZnO is 0.03:100;
[0064] Preparation method: the volume of manganese acetate solution in embodiment 1 step (1) is replaced by 67 μ L, and other parameters are all identical with embodiment 1 (at this moment the ratio of the amount of substance of manganese acetate tetrahydrate and zinc acetate dihydrate is 0.045: 100; the volume ratio of the total mass of manganese acetate tetrahydrate and zinc acetate dihydrate to deionized water is 2.7g:50mL; the mass ratio of Mn and ZnO in the mixed solution is 0.03:100), to obtain 0.03-Mn-ZnO catalyst powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com