Compound, pharmaceutical composition, medicine and application of compound, pharmaceutical composition and medicine in preparation of antibacterial products
A compound and solvate technology, applied in the application field of preparing antibacterial products, can solve problems such as adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
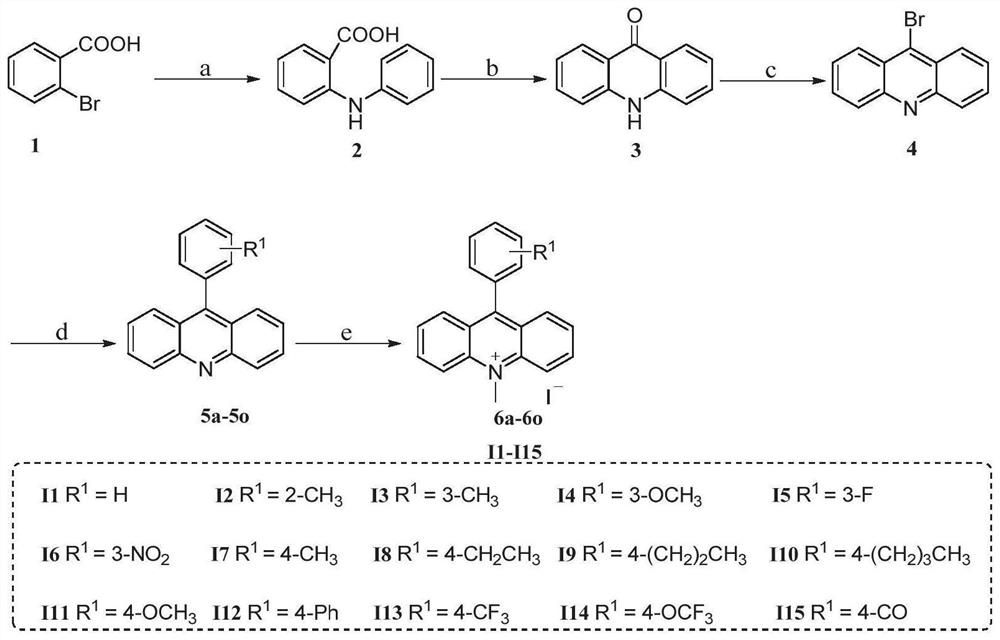
[0066] Embodiment 1: the preparation of 2-(anilino)benzoic acid (2)
[0067]
[0068] Weigh raw material 2-bromobenzoic acid (2.0g, 10mmol), aniline (1.85g, 20mmol), potassium carbonate (1.37g, 10mmol) and catalytic amount of copper powder (0.2-0.3 microns, 4.97mmol) and dissolve in 80mL in ethanol. The reaction solution was heated under reflux for 12 hours. After the reaction was monitored by TLC, the cooled reaction solution was poured into hot water, filtered through diatomaceous earth to remove insoluble matter, and then the filtrate was acidified with diluted hydrochloric acid solution to adjust the pH to 5-6, a large amount of precipitates were separated out, and the resulting precipitated product was purified by ethanol recrystallization to obtain 1.65 g of pure gray solid product, namely intermediate 2, with a yield of 78%.
Embodiment 2
[0069] Embodiment 2: Preparation of 9(10H)-acridone (3)
[0070]
[0071] The intermediate 2-(anilino)benzoic acid (2.0 g, 9.4 mmol) prepared in Example 1 above was put into a 100 mL round bottom flask, and 10 mL of concentrated sulfuric acid was added thereto. Stir the mixture under nitrogen protection at 100°C for 6 hours. After monitoring the complete reaction by TLC, cool to room temperature, and then pour the cooled reaction solution into a large amount of ice water and stir. After the solid precipitates, it is collected by suction filtration under reduced pressure. The solid was washed several times with sodium bicarbonate solution, and dried in vacuo to obtain 1.5 g of a yellow solid product, ie Intermediate 3, with a yield of 82%.
Embodiment 3
[0072] Embodiment 3: the preparation of 9-bromoacridine (4)
[0073]
[0074]The intermediate 9(10H)-acridone (4.0 g, 20.5 mmol) prepared in Example 2 above was weighed and placed in a 250 mL round bottom flask. Phosphorus tribromide (19.5 mL, 205 mmol) was injected dropwise in an ice bath at 0° C. under nitrogen protection. After the dropwise addition, the reaction solution was moved to 110° C. and stirred at this temperature for 24 hours. After the completion of the reaction was monitored by TLC, the reaction solution was cooled to room temperature, and then slowly added to water to quench the reaction. Then the filtrate was adjusted to pH 14 with sodium hydroxide solution, transferred to a separatory funnel, extracted three times with dichloromethane, the combined organic phase was washed twice with brine, dried over anhydrous sodium sulfate, filtered with suction, and extracted under reduced pressure Concentrate to obtain 3.81 g of a yellow solid product, ie Intermedi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com