Polycyclic aromatic compound as well as preparation method and application thereof
A polycyclic aromatic compound technology, applied in the field of organic optoelectronic materials, can solve the problems of narrow half-peak width, color purity, efficiency roll-off, etc., achieve narrow half-peak width, improve device life and efficiency, and avoid fluorescence quenching. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
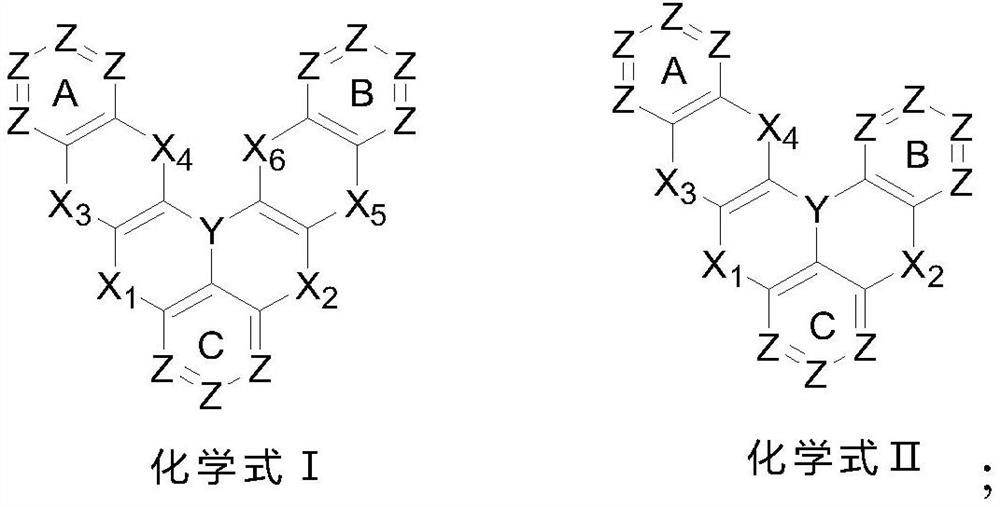
[0084] The embodiment of the present invention provides a polycyclic aromatic compound (referred to as compound 1) and its synthesis process. Specifically, compound 1 is synthesized by referring to the following synthetic route:
[0085]
[0086] The specific operation is as follows: (1) under the protection of nitrogen, add the reactant 1-1 (120mmol) and sodium tert-butoxide (220mmol) into a 1000ml three-necked flask, and dissolve it with 500ml of dry toluene solution, stir at room temperature for 10min, and then Add reactant 1-2 (120mmol), tri-tert-butylphosphine (12mmol), tris(dibenzylideneacetone) dipalladium (2mmol) under the conditions, then slowly increase the reaction temperature to 90°C, continue the reaction for 12h, use The reaction was monitored by TLC. After the reaction was completed, it was cooled to room temperature, and an appropriate amount of distilled water was added under stirring conditions, and the organic phase was retained after liquid separation. A...
Embodiment 2
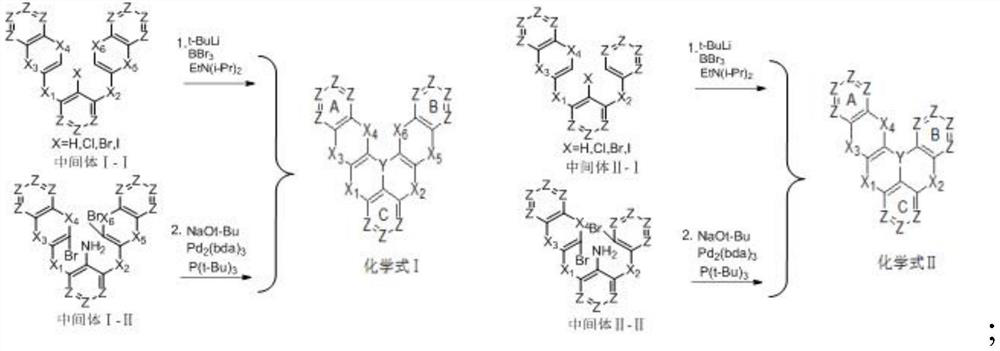
[0097] The embodiment of the present invention provides a polycyclic aromatic compound (referred to as compound 6) and its synthesis process. Specifically, compound 6 is obtained by referring to the following synthetic route:
[0098]
[0099] The specific operation is as follows: (1) under the protection of nitrogen, add the reactant 6-1 (120mmol) and sodium tert-butoxide (220mmol) into a 1000ml three-necked flask, and dissolve it with 500ml of dry toluene solution, stir at room temperature for 10min, and then Add reactant 6-2 (120mmol), tri-tert-butylphosphine (12mmol), tris(dibenzylideneacetone)dipalladium (2mmol) under the conditions, then slowly increase the reaction temperature to 90°C, continue the reaction for 12h, use TLC monitors the reaction, after the reaction is completed, cool to room temperature, add an appropriate amount of distilled water under stirring conditions, keep the organic phase after liquid separation, dry the filtrate with anhydrous magnesium sulf...
Embodiment 3
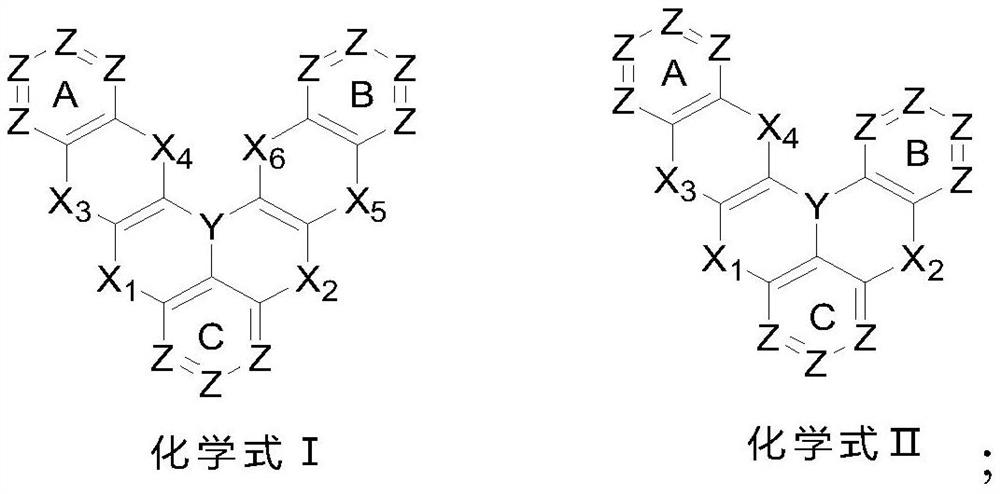
[0110] The embodiment of the present invention provides a polycyclic aromatic compound (referred to as compound 36) and its synthesis process. Specifically, compound 36 is obtained by referring to the following synthetic route:
[0111]
[0112] The specific operation is as follows: (1) Under the protection of nitrogen, add the reactant 36-1 (120mmol) and sodium tert-butoxide (220mmol) into a 1000ml three-necked flask, and dissolve it with 400ml of dry toluene solution. After stirring at room temperature for 10min, Reactant 36-2 (120 mmol), tri-tert-butylphosphine (12 mmol), and tris(dibenzylideneacetone) dipalladium (2 mmol) were added under the same conditions, and then the reaction temperature was slowly increased to 90° C., and the reaction was continued for 12 h. The reaction was monitored by TLC. After the reaction was completed, it was cooled to room temperature, and an appropriate amount of distilled water was added under stirring conditions, and the organic phase wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com