Aminohexose enzyme fluorescent probe and preparation method and application thereof
A technology of hexosaminidase and fluorescent probe is applied in the field of hexosaminidase fluorescent probe and its preparation, and achieves the effect of good stability and avoiding fluorescence quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The present invention provides the preparation method of hexosaminidase fluorescent probe described in above-mentioned technical scheme, comprises the following steps:
[0048] (1) performing a salt-forming reaction between a compound having a structure shown in formula II and a compound having a structure shown in formula III to obtain a compound having a structure shown in formula IV;
[0049]
[0050] In formula III and formula IV, R is hydrogen, alkyl, alkoxy, N,N-dimethyl, N,N-diethyl or N,N-diphenyl;
[0051] (2) The compound having the structure shown in formula IV is hydrolyzed to obtain the hexosaminidase fluorescent probe having the structure shown in formula I.
[0052] In the present invention, a compound having a structure shown in formula II is subjected to a salt-forming reaction with a compound having a structure shown in formula III to obtain a compound having a structure shown in formula IV;
[0053]
[0054]
[0055] In formula III and formula...
Embodiment 1
[0075] Prepare the hexosaminidase fluorescent probe according to the following reaction scheme:
[0076]
[0077] (1) Mix compound 1 (200mg, 0.388mmol), compound 2 (146mg, 0.310mmol) and toluene, reflux at 110°C under the protection of nitrogen, monitor the reaction process with a TLC plate until compound 2 disappears completely; the reaction mixture The insoluble impurities were removed by suction filtration, and the obtained product was concentrated to a solid by rotary evaporation, and then recrystallized with chloroform and petroleum ether. The volume ratio of petroleum ether and chloroform used was preferably 1:1 to obtain 229 mg of a yellow powdery solid.
[0078] The calculated yield is 75%;
[0079] The obtained yellow powdery solid is characterized, and the specific data are as follows:
[0080] 1 H NMR (400MHz, Chloroform-d) δ9.42(d, J=6.4Hz, 2H), 8.04(d, J=6.3Hz, 2H), 7.68–7.41(m,5H), 7.25–7.10(m, 5H), 7.10–6.83(m,8H),6.79–6.52(m,4H),6.03(q,J=13.7Hz,2H),5.62(d...
Embodiment 2
[0089] The hexosaminidase probe prepared in embodiment 1 is tested for performance, and the specific steps are as follows:
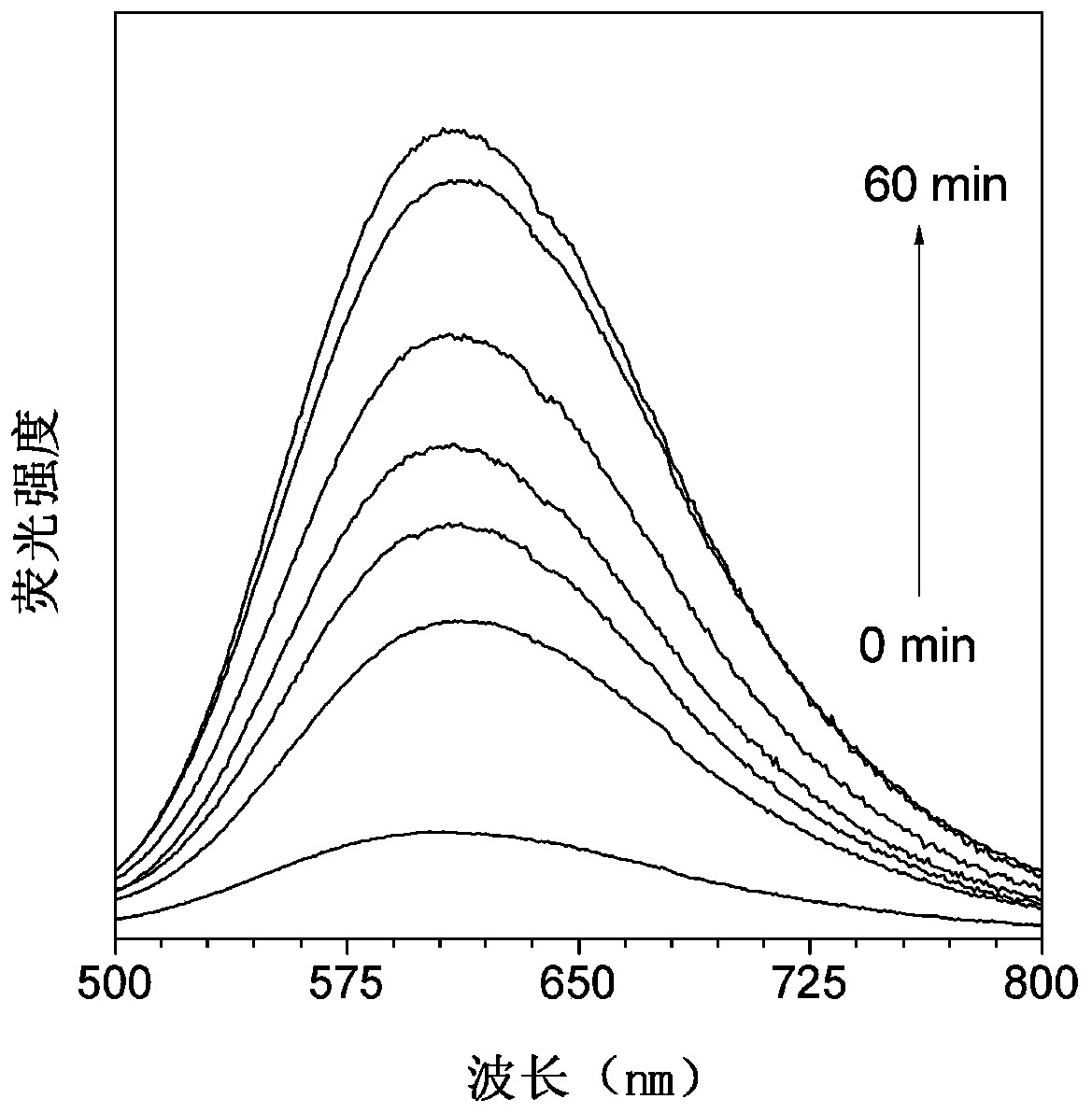
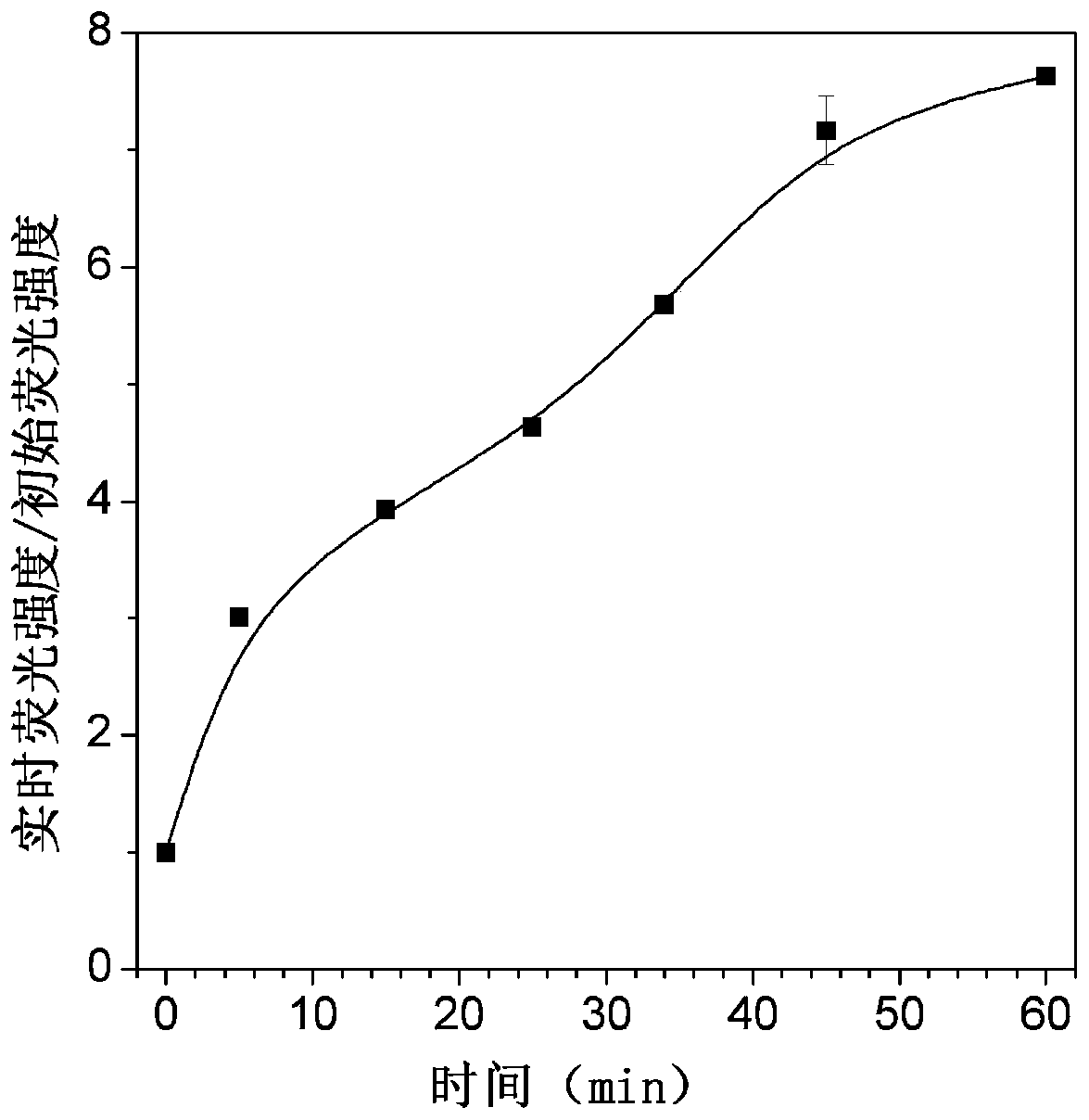
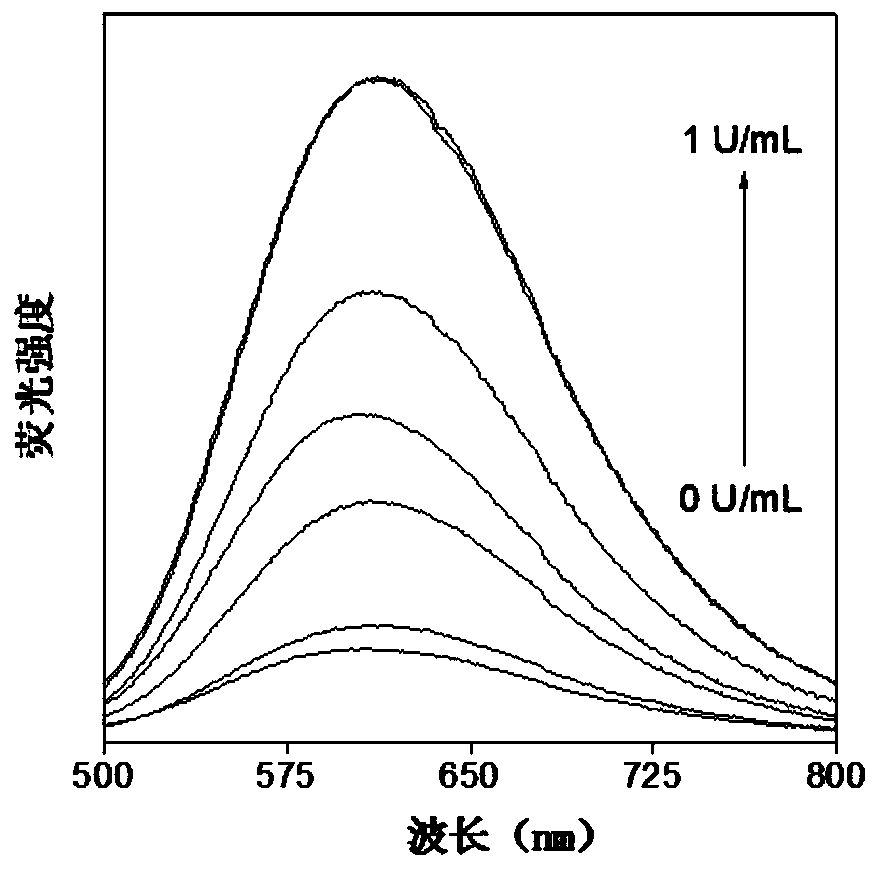
[0090] (1) Determination of the response time of the hexosaminidase probe: Add 20 μL of TPE-NAG DMSO solution (1 mM) to 2 mL of PBS solution (5 mM, pH 7.4) to obtain a 10 μM TPE-NAG solution, and then add 0.5 U / mL of hexosaminidase solution, the resulting mixed solution was incubated at 37°C for different times (0, 5, 15, 25, 35, 45, 60min), and the fluorescence spectrum of the mixed solution was measured as a function of the incubation time. Condition.
[0091] figure 1 It is the change of fluorescence spectrum of TPE-NAG after incubation in PBS for different time under the condition of 0.5U / mL hexosaminidase; figure 2 is the ratio change graph of the real-time fluorescence intensity of TPE-NAG at 612nm and the initial fluorescence intensity, by figure 1 with figure 2 It can be seen that after incubation at 37°C for 60 min, the fluorescence intens...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com