Diamine monomer containing carborane structure, dianhydride monomer containing carborane structure and preparation method and application of diamine monomer and dianhydride monomer containing carborane structure
A diamine monomer and carborane-containing technology, which is applied in the field of polyimide monomers containing carborane structure and its preparation, can solve the problem that the preparation technology of dianhydride monomers and diamine monomers is immature and unable to To meet the requirements of mass production and other problems, to achieve the effect of high yield, convenient raw materials, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] Another aspect of the embodiments of the present invention also provides a method for preparing a diamine monomer containing a carborane structure, which includes:
[0077] (1) Adding the first uniformly mixed reaction system comprising decaborane, acetonitrile and the first solvent at 80-120° C. for 3-6 hours to prepare a complex of decaborane and acetonitrile;
[0078] (2) The second homogeneously mixed reaction system comprising the complex of decaborane and acetonitrile, the alkynyl compound, silver nitrate and the second solvent is reacted at 80-120°C for 3-8 hours, and then obtained by post-treatment Compounds containing carborane structures;
[0079] (3) The third homogeneously mixed reaction system comprising the compound containing the carborane structure, concentrated nitric acid and concentrated sulfuric acid is subjected to nitration reaction at 0-60°C for 4-10 hours to obtain a nitro compound containing the carborane structure ;
[0080] (4) The fourth un...
Embodiment 1
[0144] Step 1: Add decaborane (122.3g) and acetonitrile (82.1g) into 1L of toluene and mix, heat to reflux at 80°C for 4h, after the reaction is completed, distill under reduced pressure and wash (washed twice with cyclohexane) , the white solid (0.99mol, productive rate 99%) obtained after drying is the complex compound of decaborane and acetonitrile;
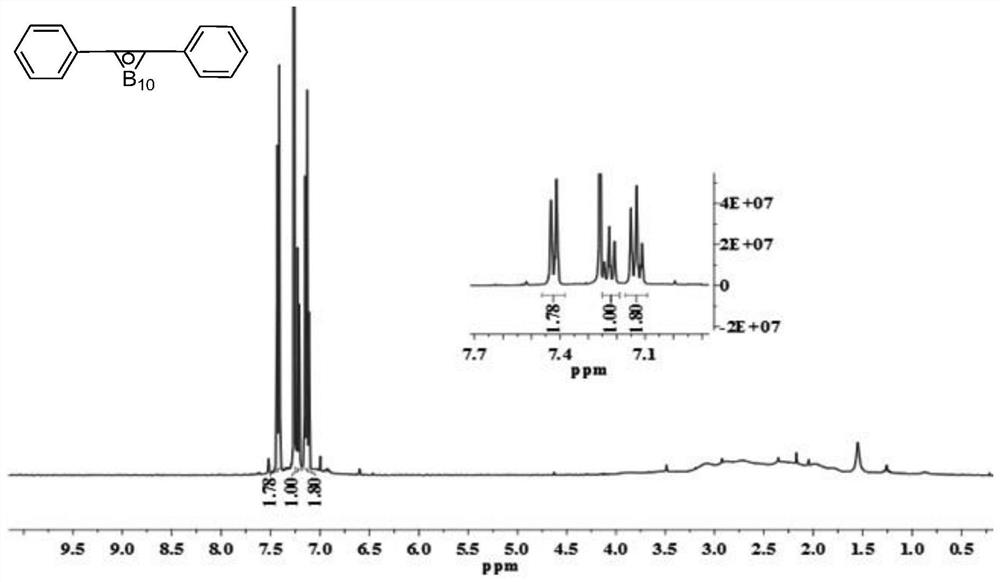
[0145] Step 2: Add toluene (160.4g), the complex of decaborane and acetonitrile (0.99mol) obtained in Step 1, and silver nitrate (0.45mol) into 1.5L of toluene and mix, and heat to reflux at 100°C 8h, after the reaction was completed, the white solid (219.1g, 0.73mol, yield 81%) obtained after vacuum distillation, washing (sequentially with methanol 2 times, hydrochloric acid 2 times, and water 3 times) and drying was carbon-containing boron Compounds with alkane structure (NMR spectrum see figure 1 );
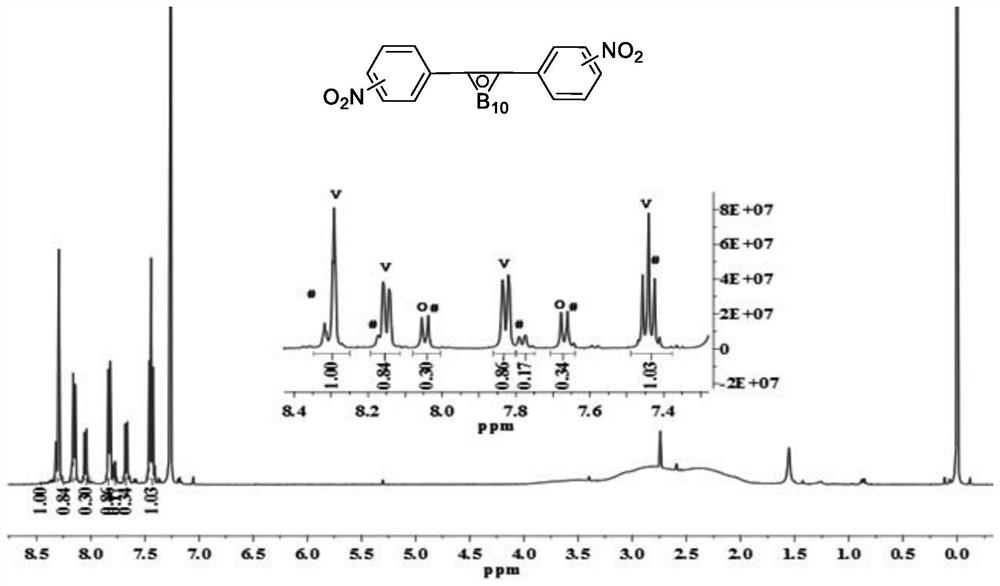
[0146] Step 3: Add the carborane-containing compound (0.5 mol) obtained in Step 2 into a mixture of concentrated nitric ac...
Embodiment 2
[0150] Step 1: Add decaborane (122.3g) and acetonitrile (82.1g) into 1L of toluene and mix, then heat and reflux at 120°C for 4h, after the reaction is completed, distill under reduced pressure, wash (washed twice with cyclohexane), The white solid obtained after drying (0.99mol, yield rate 99%) is the complex of decaborane and acetonitrile;
[0151] Step 2: In an ice-water bath, slowly add di-tert-butyl dicarbonate (523.8g) dropwise to a dichloromethane solution containing an alkynyl-containing diamine compound (222.3g), and stir the reaction at room temperature after the dropwise addition is complete After 10 h, the white solid (0.99 mol, yield 99%) obtained by distillation and washing is the Boc-protected alkyne-containing diamine compound;
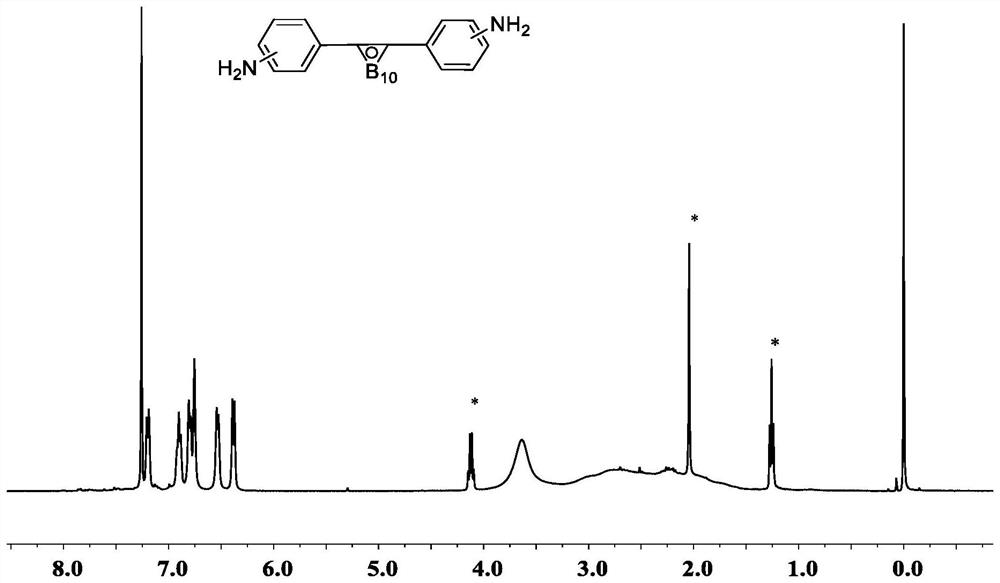
[0152] Step 3: Add the Boc-protected alkynyl-containing diamine compound (380.3g) obtained in Step 3, the complex (199.8g) of decaborane and acetonitrile obtained in Step 1, and silver nitrate (15.3g) to 1.5L of toluene , heated to re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com