Method for analyzing lamotrigine by high performance liquid chromatography
A high-performance liquid chromatography and lamotrigine technology, applied in the field of drug analysis, can solve the problems of emphasizing process impurities or degrading impurities, affecting the accuracy of impurities, and clogging the liquid phase system, so as to achieve short running time, cost saving, and baseline gentle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
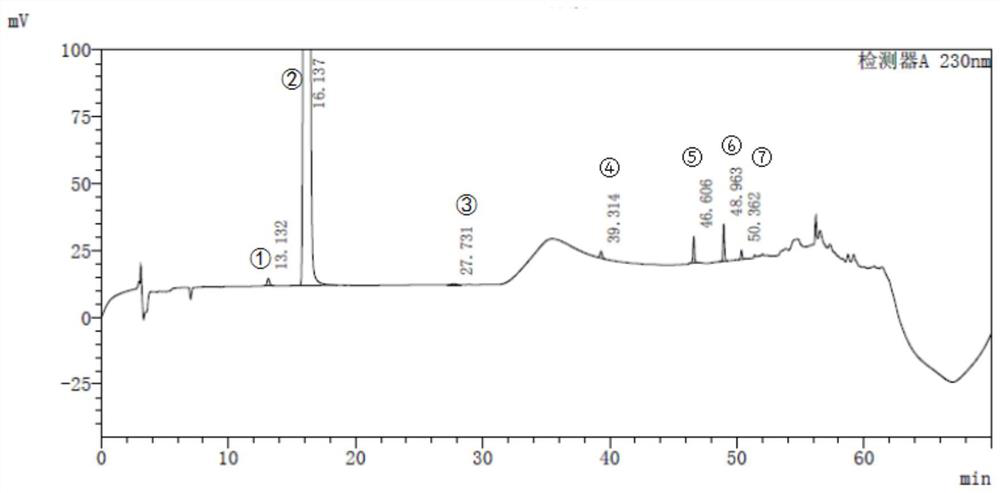
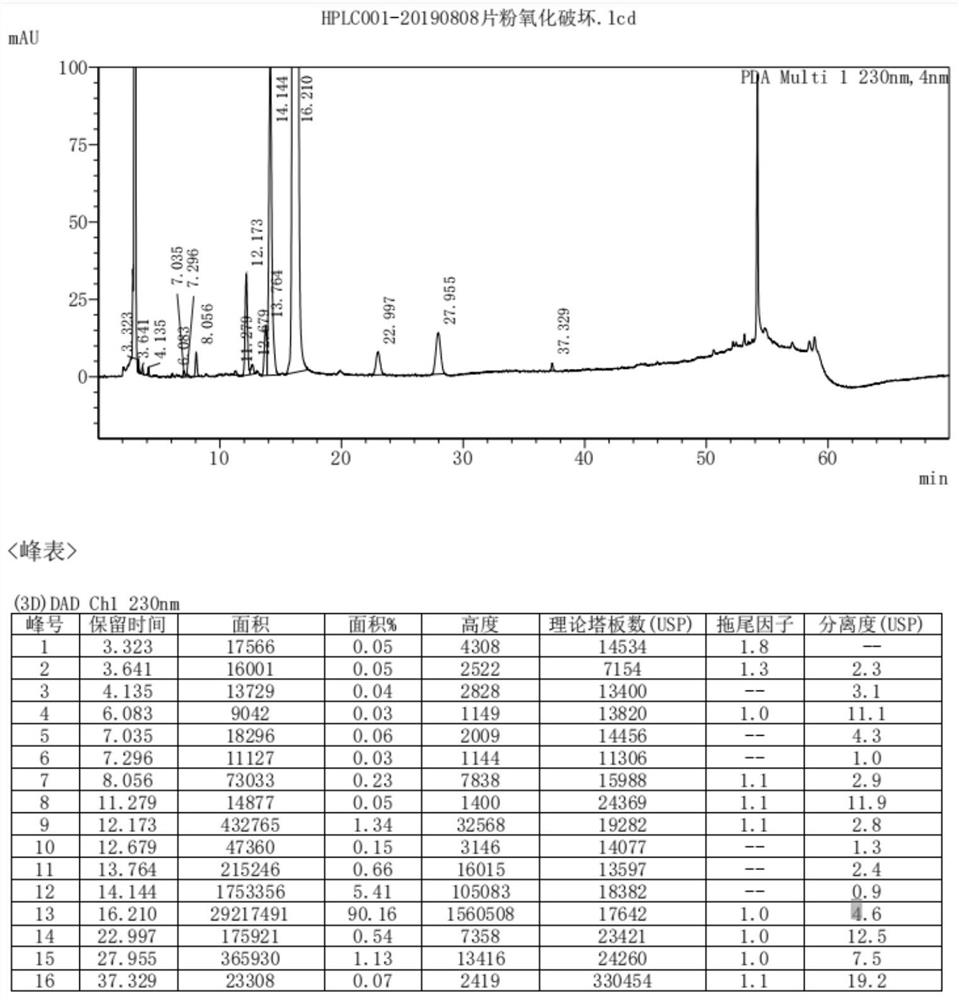
[0027] Chromatographic conditions
[0028] In a typical embodiment of the invention, the chromatographic conditions are:
[0029] Chromatographic column: octadecylsilane bonded silica gel column, 4.6×250mm, 5μm;
[0030] Mobile phase A: Methanol;
[0031] Mobile phase B: 0.5% triethylamine solution adjusted to pH 4.5 with phosphoric acid;
[0032] Flow rate: 0.8ml / min;
[0033] Wavelength: wavelength, 230nm;
[0034] Column temperature: 40°C;
[0035] Injection volume: 20μl;
[0036] Gradient program: Table 2.
[0037]
[0038] The preparation of test sample and reference substance solution
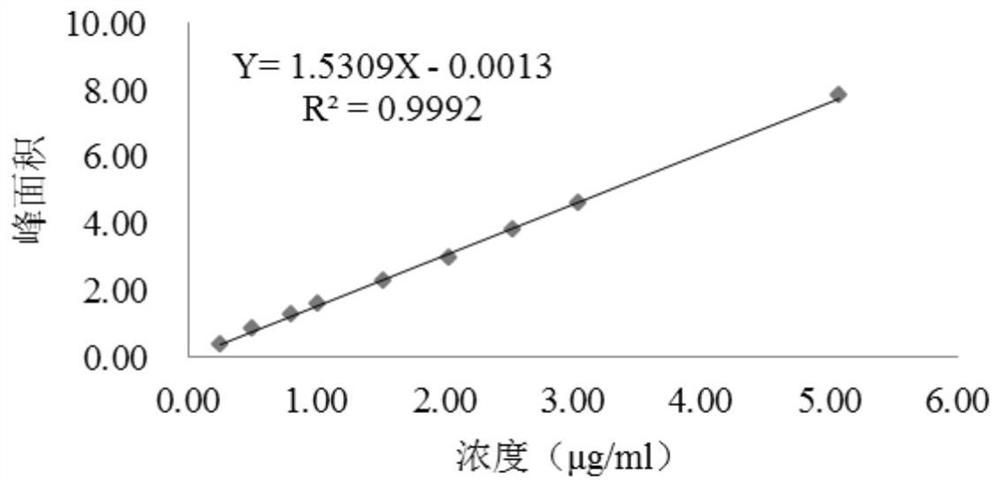
[0039] For preparing need testing solution and reference substance solution, the diluent that the embodiment of the present invention uses is methanol-0.1mol / L hydrochloric acid solution (20:80) mixed solution. Tests have shown that samples diluted with this diluent are also stable at room temperature for 24 hours.
[0040] To prepare the test solution, take an appropriate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com