Non-stoichiometric lithium iron manganese phosphate positive electrode material as well as preparation method and application thereof
A non-stoichiometric technology of lithium manganese iron phosphate, which is applied in the field of lithium-ion batteries, can solve problems such as difficult control and sensitive synthesis conditions, and achieve easy control of the process, improvement of electrochemical performance, discharge specific capacity and cycle stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Lithium dihydrogen phosphate (2.0786g), ferrous oxalate dihydrate (1.7989g), manganese carbonate (1.1495g), lithium carbonate (0.0369g), sucrose (0.1885g), polyvinyl alcohol (0.1885g) Place in a zirconia ball mill jar filled with 30 g of absolute ethanol and 1.5 g of oleic acid, and ball mill at a speed of 400 r / min for 8 hours to obtain a suspension;
[0050] (2) Centrifuge the suspension in step (1), and vacuum-dry the obtained solid mixture at 80° C. for 1 hour to obtain a precursor;
[0051] (3) The precursor powder obtained in step (2) was placed in a tube furnace with an argon atmosphere, the temperature was raised to 420° C. for pre-calcination for 5 hours, and then the temperature was raised to 650° C. for 8 hours. get Li 1.05 Fe 0.5 mn 0.5 PO 4 / C cathode material (the non-stoichiometric lithium manganese iron phosphate cathode material).
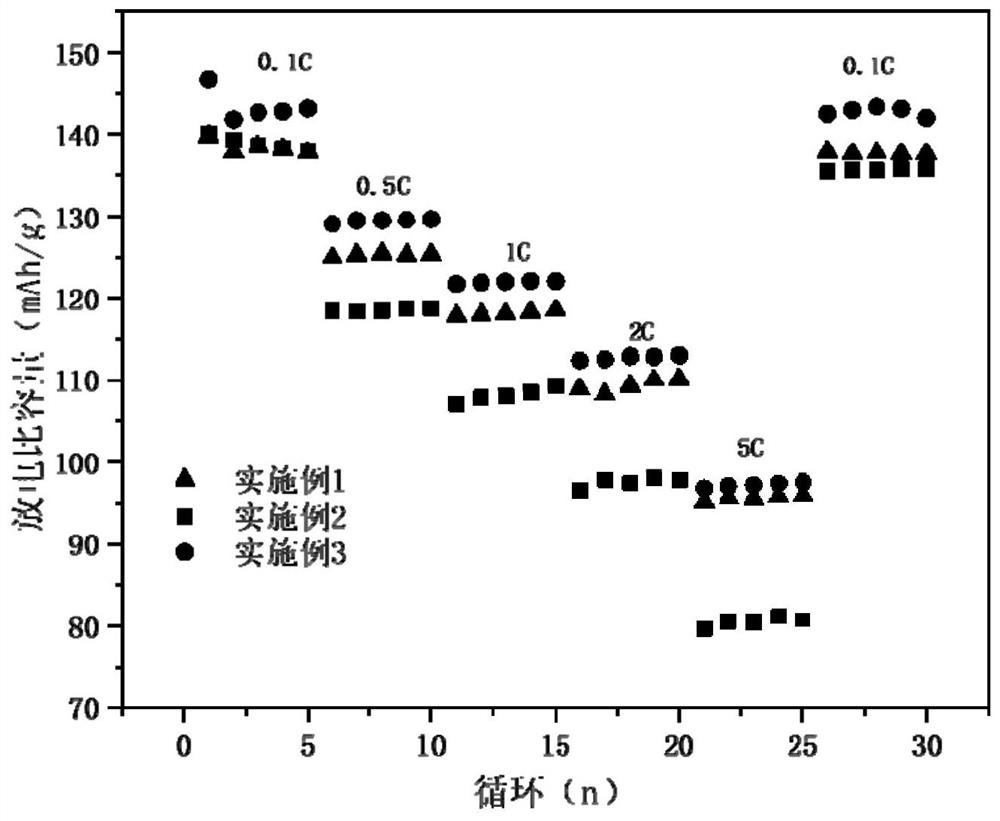
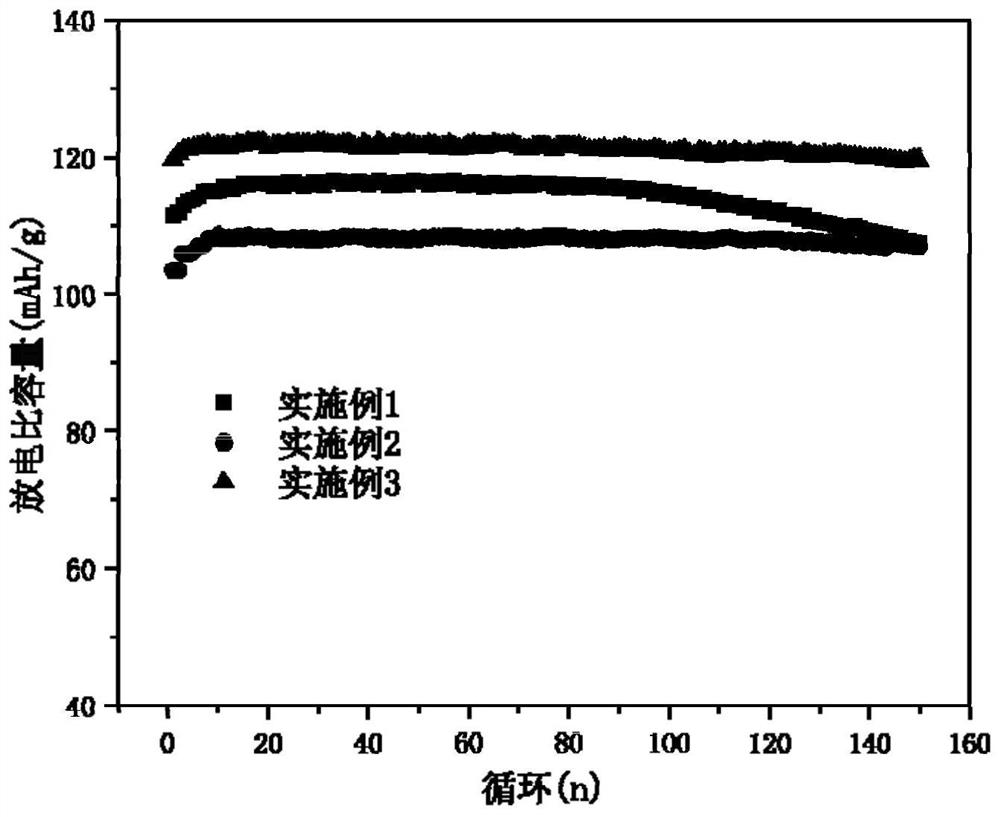
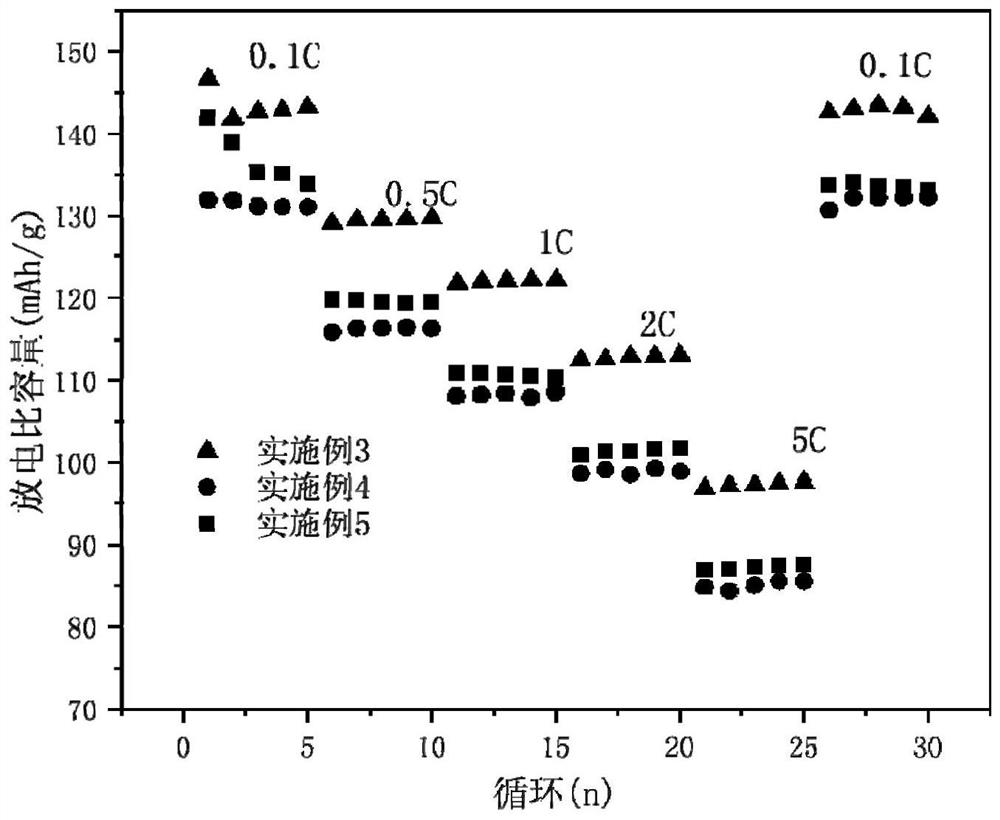
[0052] Such as figure 1 Shown is the rate performance diagram of the material prepared in Example 1 under differ...
Embodiment 2
[0057] (1) Lithium dihydrogen phosphate (2.0786g), ferrous oxalate dihydrate (1.7089g), manganese carbonate (1.1495g), lithium carbonate (0.0369g), sucrose (0.1885g), polyvinyl alcohol (0.1885g) Place in a zirconia ball mill jar filled with 30 g of absolute ethanol and 1.5 g of oleic acid, and ball mill at a speed of 400 r / min for 8 hours to obtain a suspension;
[0058] (2) Centrifuge the suspension in step (1), and vacuum-dry the obtained solid mixture at 80° C. for 1 hour to obtain a precursor;
[0059] (3) The precursor powder obtained in step (2) was placed in a tube furnace with an argon atmosphere, the temperature was raised to 420° C. for pre-calcination for 5 hours, and then the temperature was raised to 650° C. for 8 hours. get Li 1.05 Fe 0.475 mn 0.5 PO 4 / C cathode material (the non-stoichiometric lithium manganese iron phosphate cathode material).
[0060] Such as figure 1 Shown is the rate performance diagram of the material prepared in Example 2 under diff...
Embodiment 3
[0065] (1) Lithium dihydrogen phosphate (2.0786g), ferrous oxalate dihydrate (1.7989g), manganese carbonate (1.0920g), lithium carbonate (0.0369g), sucrose (0.1885g), polyvinyl alcohol (0.1885g) Place in a zirconia ball mill jar filled with 30 g of absolute ethanol and 1.5 g of oleic acid, and ball mill at a speed of 400 r / min for 8 hours to obtain a suspension.
[0066] (2) The suspension in step (1) was centrifuged, and the obtained solid mixture was vacuum-dried at 80° C. for 1 hour to obtain a precursor.
[0067] (3) The precursor powder obtained in step (2) was placed in a tube furnace with an argon atmosphere, the temperature was raised to 420° C. for pre-calcination for 5 hours, and then the temperature was raised to 650° C. for 8 hours. get Li 1.05 Fe 0.5 mn 0.475 PO 4 / C cathode material (the non-stoichiometric lithium manganese iron phosphate cathode material).
[0068] Such as figure 1 As shown, the first discharge specific capacity of the material prepared in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com