Construction of nano-carrier for regulating adaptive cell and humoral immunity and application thereof
A technology of adaptive cells and nano-carriers, which is applied in the direction of antigen carriers, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of high preparation costs, lack of targeting methods, and unfavorable scale-up production, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Prescription Screening, Antigen Loading and Cell Uptake of Cationic Nanoemulsion
[0048] (1) Prescription screening and physical and chemical properties characterization of blank nanoemulsion
[0049] Table 1: Recipe composition of nanoemulsion
[0050]
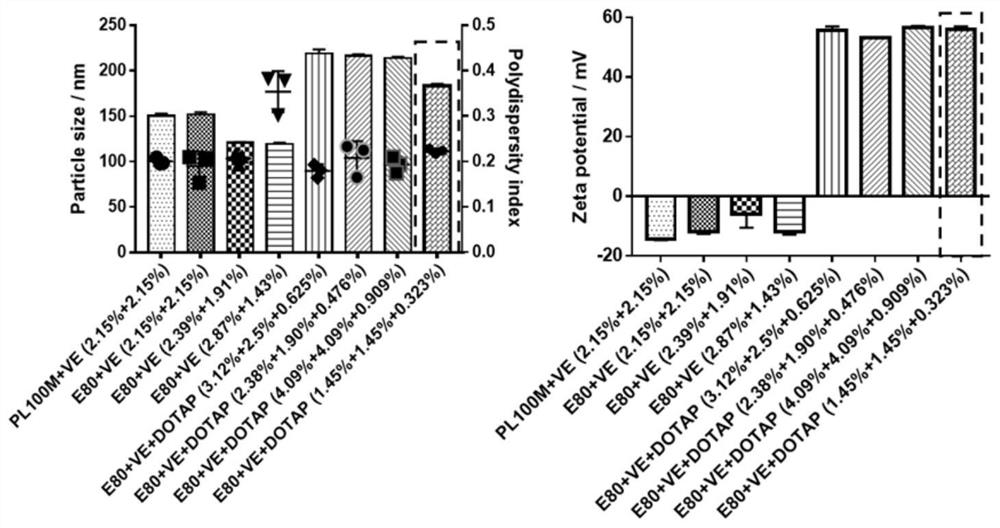
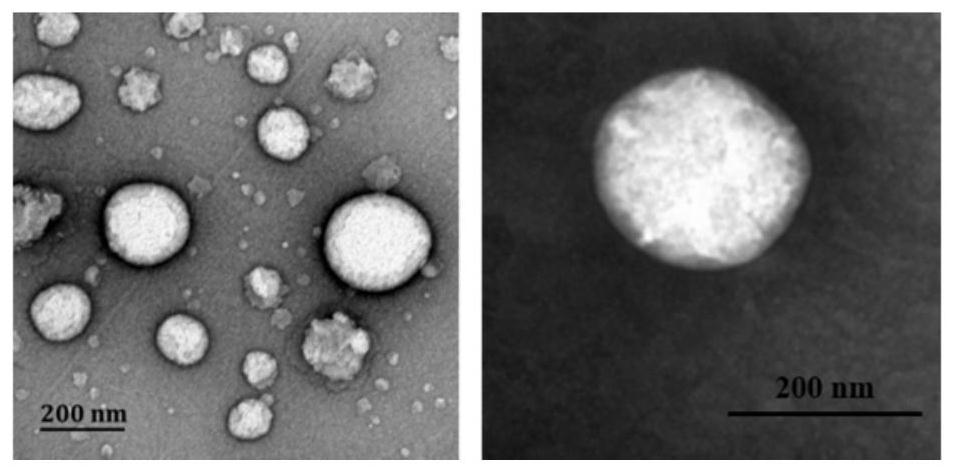
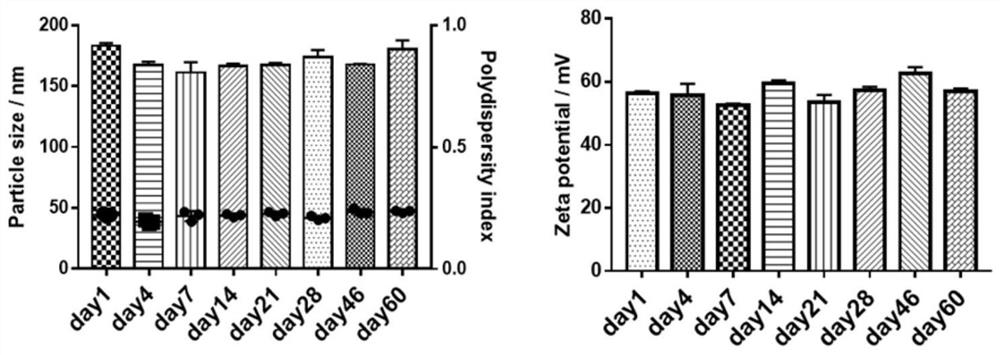
[0051] First, a blank nanoemulsion was prepared by emulsification ultrasonic method, and its prescription was screened. By changing the type of PC (PL100M and E-80), the proportion of each lipid component and the content of the water phase, a prescription with higher stability is screened out, namely prescription 8 (E80:VE:DOTAP=1.45%:1.45% :0.323%, w / w), for follow-up research (see figure 1 ). The nanoemulsion particle diameter under this prescription is about 200nm, and potential is about 60mV (dynamic light scattering method detects); Under transmission electron microscope (TEM), present typical emulsion form (see figure 2 ); high stability and easy storage, there is no significant change in parti...
Embodiment 2
[0056] Example 2 Preparation of cationic nanoemulsion loaded with model antigen OVA and surface modified p-dodecylbenzenesulfonamide and characterization of its endoplasmic reticulum targeting
[0057] (1) Endoplasmic reticulum-targeted surface modification of antigen-loaded cationic nanoemulsions
[0058] Composition of endoplasmic reticulum-targeted antigen-loaded cationic nanoemulsions
[0060] Vitamin E (VE) 9mg
[0061] (2,3-dioleoyl-propyl)trimethylammonium chloride (DOTAP) 1mg~28mg
[0062] p-dodecylbenzenesulfonamide 1mg~8mg
[0063] Aqueous solution of OVA (5 mg / mL) 0.6 mL.
[0064] In order to better realize the loading of the antigen and the targeted accumulation of the endoplasmic reticulum, the prescription 8 in the implementation example 1 was further adjusted: the aqueous solution of OVA was directly used as the water phase, and the oil phase of the cationic nanoemulsion was added with Endoplasmic reticulum tropism of p-dodecy...
Embodiment 3
[0067] Example 3 Verification of Endoplasmic Reticulum-targeted Antigen-Loaded Cationic Nanocarriers Improving Cellular Immunity and Humoral Immunity
[0068] Directly dissolving the antigenic protein in the aqueous phase of the emulsion is conducive to the internal loading and external adsorption of the antigen during the assembly process of the carrier, which can further increase the drug loading capacity of the antigen, promote the cellular uptake of the antigen, and thus up-regulate the cellular immunity mediated by DCs and overall responsiveness of humoral immunity. The endoplasmic reticulum targeting properties of the carrier are conducive to the selective accumulation of antigens in the endoplasmic reticulum of DCs, and mechanisms such as endoplasmic reticulum-related degradation can be used to promote the MHC class I presentation of exogenous antigens, thereby increasing the participation of cellular immunity.
[0069] In order to verify the advantages of this endoplas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com