Method for inducing adipose tissue-derived stem cells to differentiate into testicular interstitial-like cells by single transcription factor
A technology of stromal stem cells and testicular interstitium, applied in the field of stem cell biology and regenerative medicine, to achieve the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Isolation and cultivation of human-derived ADMSCs.
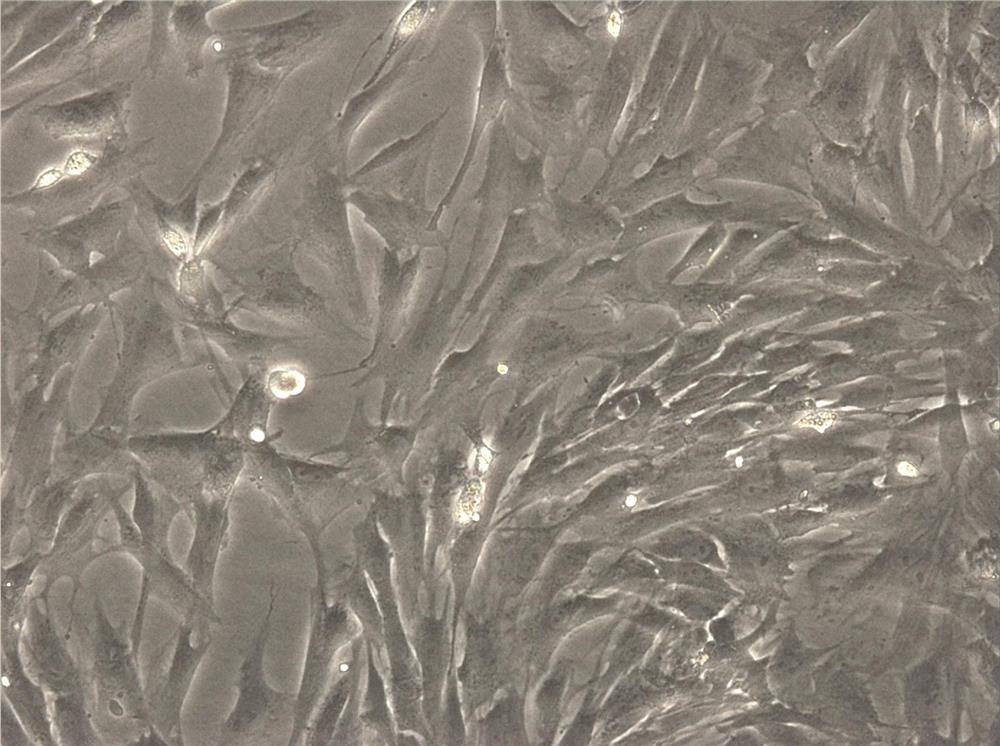
[0032] Collect the resected tissue after circumcision in the operating room, remove the skin and retain the fat, cut it into pieces with sterile ophthalmic scissors, incubate with 0.2% collagenase I solution at 37°C for 1-2 hours, and culture with DMEM containing 10% fetal bovine serum Stop the digestion, filter aseptically, centrifuge at 250×g for 5 minutes, discard the supernatant, resuspend the bottom of the tube, centrifuge again, and resuspend the cells in DMEM medium containing 1% penicillin and streptomycin and 10% fetal bovine serum , inoculated into a culture dish, replaced with fresh culture medium after overnight culture, subcultured, and retained the third generation cells ( figure 1 ) were stored frozen at -80°C for later use.
Embodiment 2
[0033] Example 2. Optimal synthesis of modNR5A1 in vitro.
[0034] In the case of avoiding nuclease contamination, first use the pTEMPlz plasmid (purchased from Promega, USA) and 2×HiFi HotStart Ready Mix kit (purchased from Kapa biosystems, USA) to synthesize poly T-tailed DNA by Tail-PCR reaction. Linear DNA template (see Kondrat et al., Methods in molecular biology, 2017, 1521: 127-138). i.e. first pass AleI, AfeI Enzymes linearize the pTEMPlz plasmid, use a pair of primers (1 μM) of the NR5A1 gene and 2× KAPA HiFi HotStart ReadyMix (KAPA Biosystems, USA) to amplify its open reading frame (ORF) by PCR reaction, and use T4 DNA ligation The enzyme clones the amplified ORF into the pTEMPlz plasmid vector, and then transforms, screens positive clone colonies for resistance, and shakes the bacteria to amplify and extract the plasmid. The concentration is detected by the NanoDrop instrument and adjusted to 100-200 ng / μl, and placed in - Store at 80°C for later use.
[0035] Usi...
Embodiment 3
[0040] Example 3. Extracting urine-derived exosomes and loading them with modNR5A1 molecules.
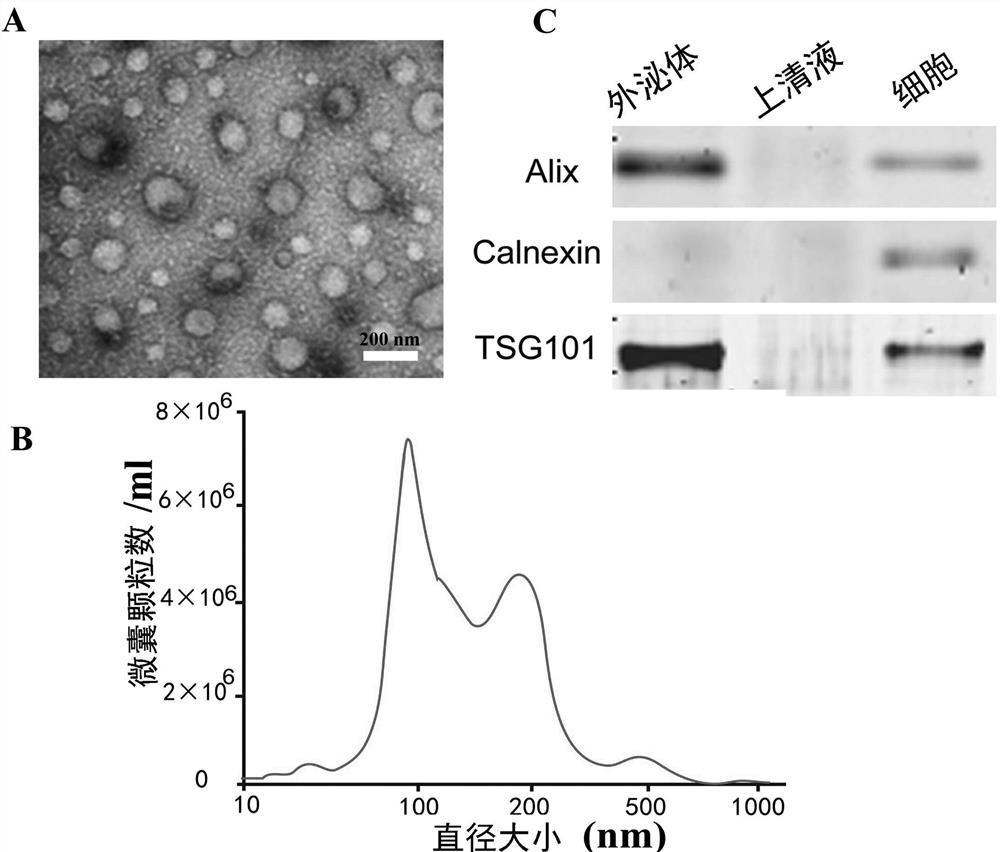
[0041] Collect autologous urine from circumcision patients, and separate exosomes in a 50ml sterile centrifuge tube at 4°C by ultracentrifugation. First, centrifuge at 2,000×g for 20 minutes to remove cells and debris, and then centrifuge at 10,000×g for 60 minutes to remove microcapsules. , and finally centrifuged at 100000×g for 2 hours; next, observe the shape and size of the extract by electron microscope ( figure 2 A), nanoparticle tracking analysis (Nanoparticle Tracking Analysis, NTA) to detect the molecular size of the extract ( figure 2 B), Western blotting method to detect the expression of exosomes markers ( figure 2 C), confirming that the extracts are exosomes.
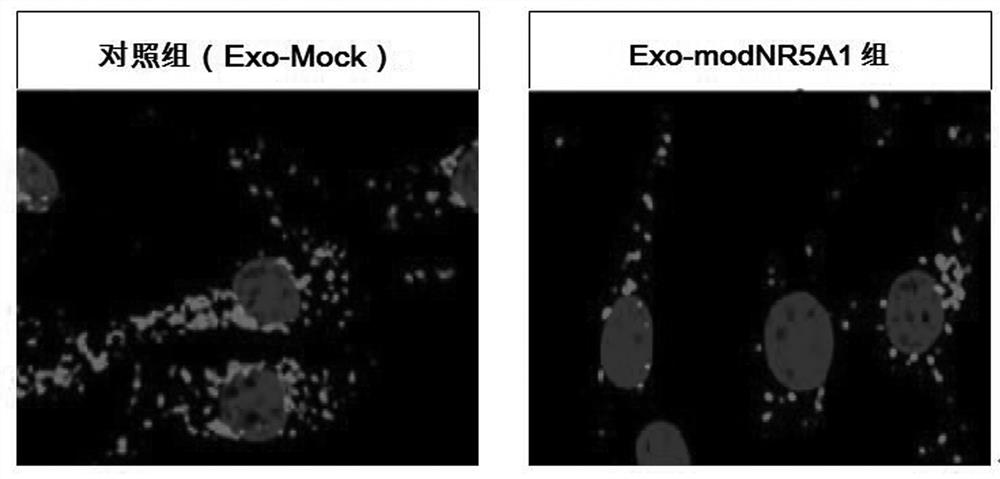
[0042] The extracted exosomes were placed on ice, mixed with the fluorescent probe PKH26 dye and incubated for 12 hours, so that the exosomes were labeled with PKH26. Then, through optimized sonication: 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com