Phase-change nano composite material with bubbles promoting drug release, preparation method and application
A nanocomposite material and composite material technology are applied in phase change nanocomposite materials, synergistic treatment application of tumor photothermal therapy and rapid chemotherapy, and preparation field, and can solve the complex preparation method, high cost, poor stability and morphology uniformity. and other problems, to achieve the effect of high repeatability, small particle size and good morphology uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
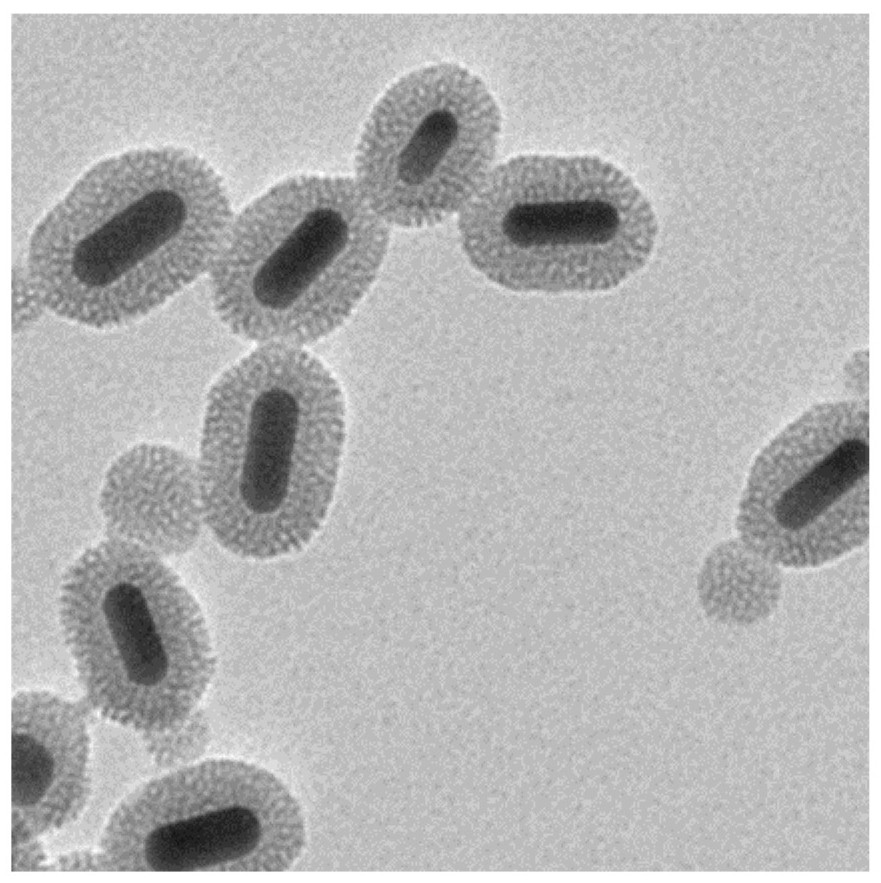
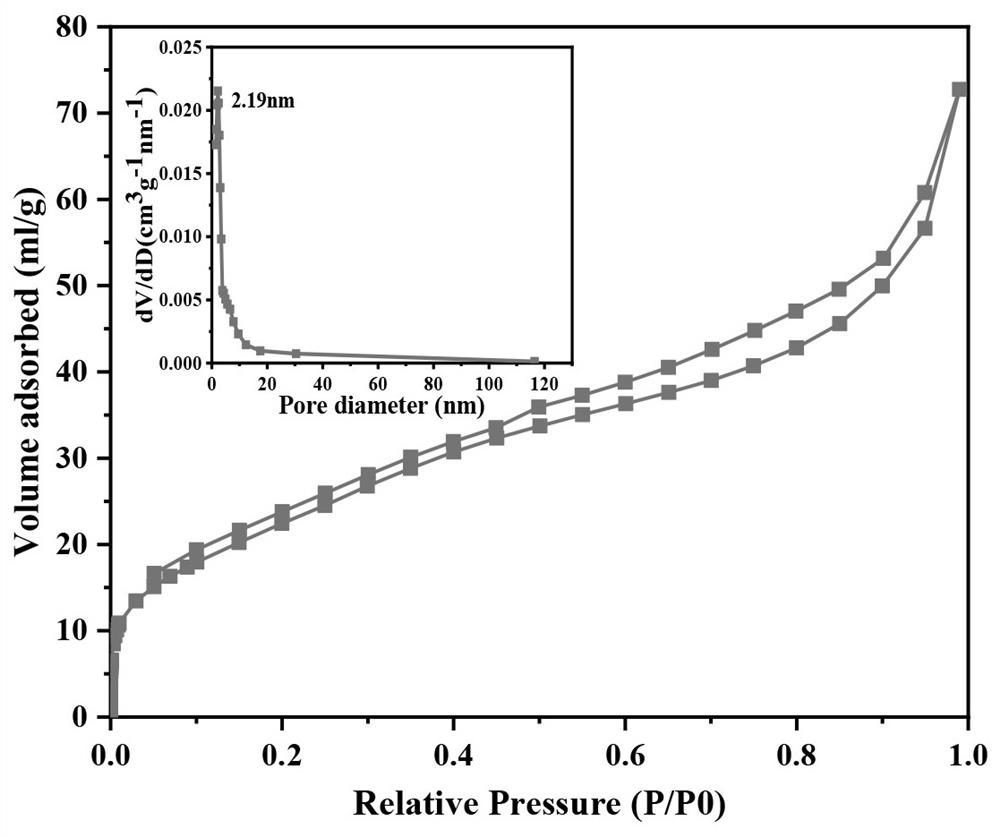
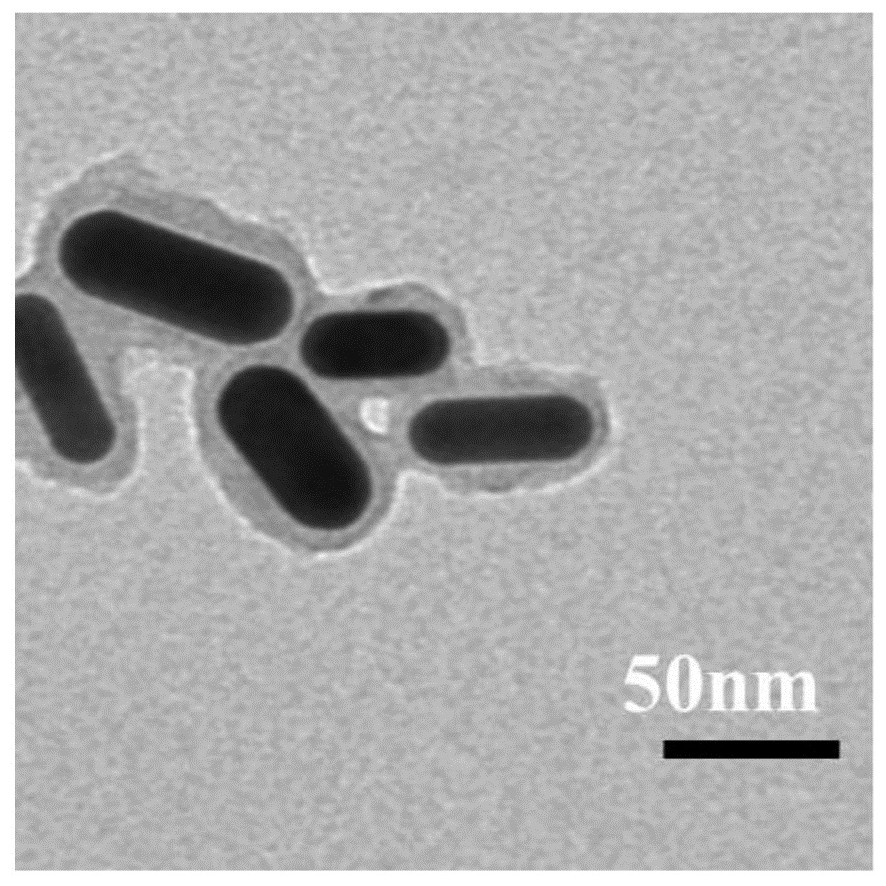
[0043] This example provides a bubble-promoted phase-change nanocomposite material for drug release. The nanomaterial uses mesoporous silica with an ordered pore structure to coat gold nanorods GNR@mSiO 2 As a carrier, the temperature-sensitive phase change material and doxorubicin hydrochloride DOX are loaded in the pore structure of the nanoparticle to obtain the composite material PFP@GNR@mSiO 2 -DOX, and its outermost layer is coated with a biocompatible polydopamine layer to prevent drug leakage, resulting in FPF@GNR@mSiO 2 -DOX@PDA composite material; the particle shape of the nanomaterial is uniform, it has photothermal effect, good water solubility and biocompatibility.
[0044] In the composite FPF@GNR@mSiO 2 -DOX@PDA finished dry matter, the temperature-sensitive phase change material is perfluoro-n-pentane, and its mass ratio is 3.0%~5.0%, which is 3% in this embodiment; the mass of doxorubicin hydrochloride DOX accounts for The ratio is 4.0%~5.0%, which is 4% in ...
Embodiment 2
[0053] The bubble-promoted drug release phase-change nanocomposite material, preparation method and application provided in this example are basically the same as in Example 1, except that the composite material FPF@GNR@mSiO 2 -DOX@PDA finished product dry matter, the temperature-sensitive phase change material is perfluoro-n-pentane, its mass proportion is 4.0%; doxorubicin hydrochloride DOX mass proportion is 4.5%.
[0054] The preparation method of the bubble-promoted drug release phase-change nanocomposite provided in this embodiment, and further preparing it into a gold nanorod-based phase-change nano-medicine composite material, includes the following steps:
[0055] (1) Use the seed-mediated method to prepare seeds: take 4.88mL cetyltrimethylammonium bromide (CTAB, 0.1mol / L) and 0.12mL HAuCl 4 ٠3H 2 O (0.01mol / L) was stirred for 10min; then 0.3mL sodium borohydride (0.01mol / L) was added, and mixed well, ready for use. Add 190mL of CTAB (0.1mol / L) solution, 10mL of HAu...
Embodiment 3
[0067] The bubble-promoted drug release phase-change nanocomposite provided in this example, its preparation method and its application are basically the same as those in Examples 1 and 2, except that:
[0068] In the composite FPF@GNR@mSiO 2 -DOX@PDA finished product dry matter, the temperature-sensitive phase change material is perfluoro-n-pentane, which accounts for 5.0% by mass; doxorubicin hydrochloride DOX accounts for 5.0% by mass.
[0069] In the step (1) of the preparation method, for each centrifugation step involved, the centrifugation speed is 13500 r / min, and the centrifugation time is 20 minutes; add an equal volume of deionized water and centrifuge again, the centrifugation speed is 11200 r / min, centrifugation Time 20min, dispersed in 20mL of deionized water.
[0070] In this example, the prepared gold nanorods and nanomaterials modified by mesoporous silicon (GNR@mSiO 2 ), a method applied to photothermal experiments, comprising the following steps:
[0071]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com