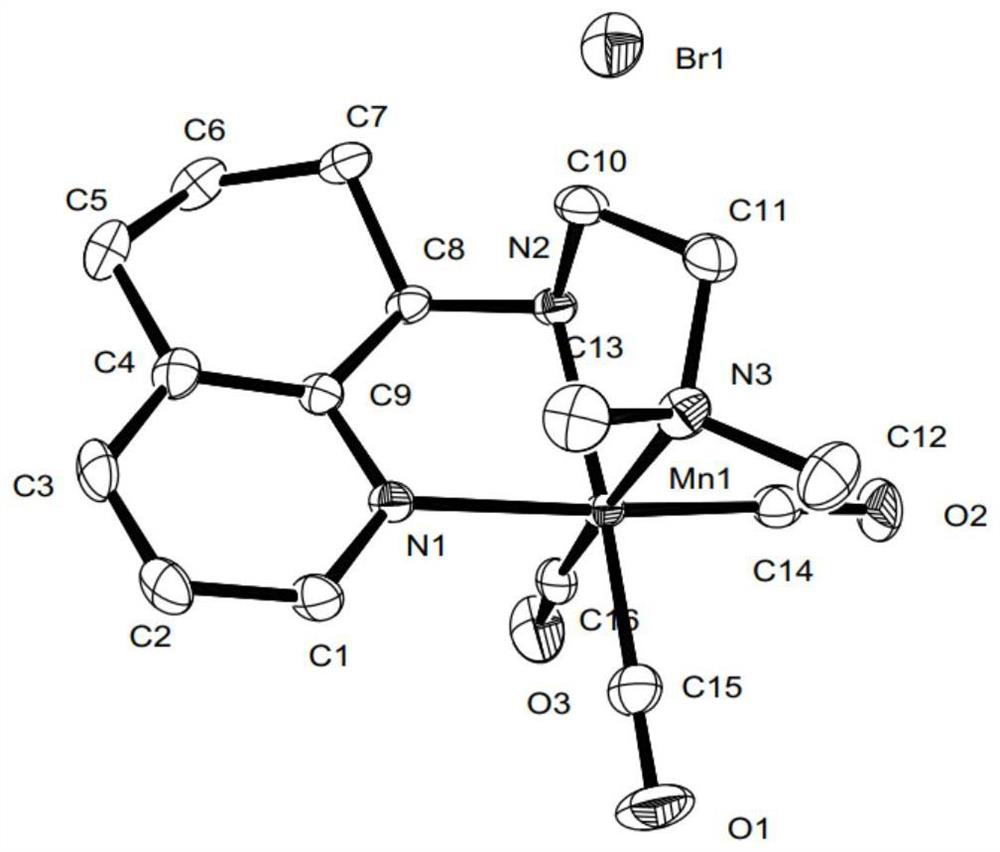
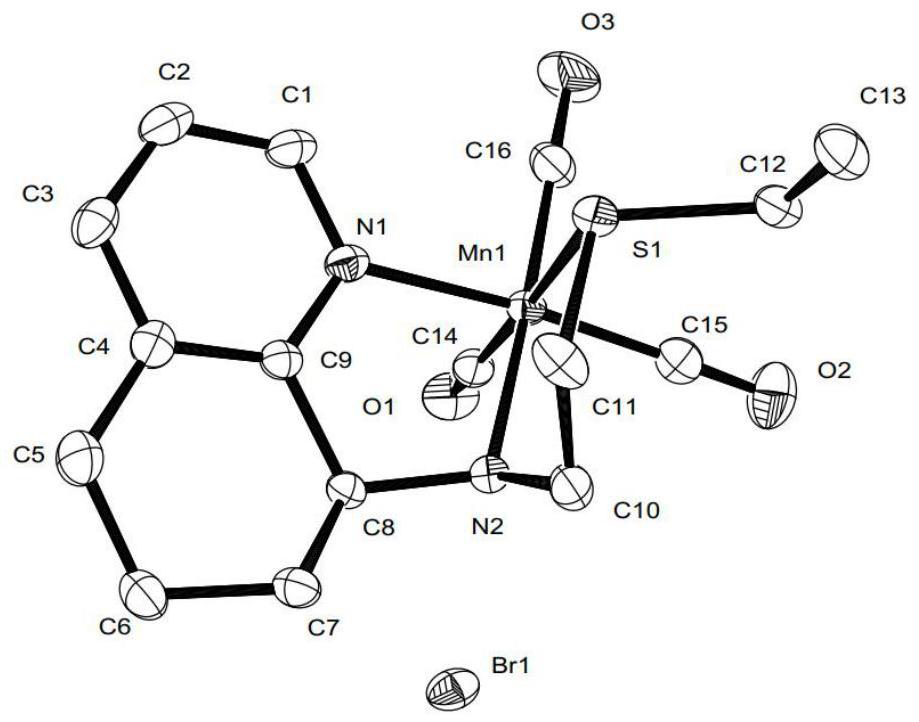
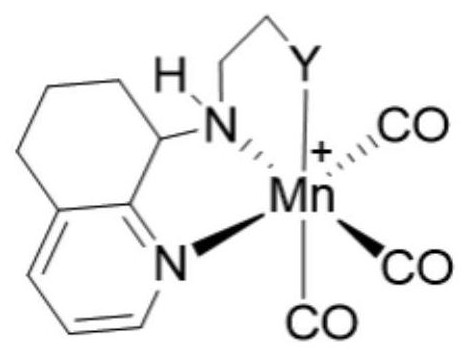
Pincerlike manganese complex and preparation method thereof, related ligand and preparation method thereof, catalyst composition and application
A technology of manganese complexes and catalysts, which is applied in the field of catalyst compositions, can solve the problems of unsuitability for large-scale industrial production, excessive use of additives, and low activity of manganese catalysts, so as to improve catalytic activity and substrate applicability with low cost , the effect of a wide range of substrate application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] Using a similar preparation method and molar ratio to L1 in Example 1, 3.50 g of L2 was obtained as a yellow oil with a yield of 70%.
[0049] The structural confirmation data are as follows:
[0050] 1 H NMR (CDCl 3 ,400MHz)δ8.30(dd,J=4.8,1.6Hz,1H),7.30(dd,J=7.7,1.6Hz,1H),6.99(dd,J=7.7,4.7Hz,1H),5.18(s ,1H,NH),3.78(dd,J=8.1,5.4Hz,1H),2.82–2.65(m,4H),2.65–2.58(m,2H),2.52 (q,J=7.1Hz,4H), 2.16–2.07(m,1H),1.98–1.90(m,1H),1.81–1.60(m,2H),0.97(t,J=7.1Hz,6H,CH 3 ); 13 C NMR (CDCl 3,100MHz) δ156.51, 146.77, 136.97, 132.52, 122.00, 57.83, 52.30, 46.88, 44.38, 28.72, 28.18, 19.77, 11.38.
[0051] The crude product L2 can be acidified by adding 10 mL of 10w% HCl aqueous solution, and further purified to obtain 4.85 g of light yellow solid ligand hydrochloride, with a total yield of 68%.
[0052] The structural confirmation data are as follows:
[0053] 1 H NMR (D 2 O, 400MHz) δ8.54 (d, J = 5.2Hz, 1H), 8.13 (d, J = 7.9Hz, 1H), 7.71 (ddt, J = 7.8, 5.1, 2.6Hz, 1H), 4.58 (d...
Embodiment 4
[0062] Example 4 Preparation of 2-(ethylthio)ethylamine
[0063]
[0064] Under nitrogen atmosphere, 11.3g (0.10mol) 2-chloroethylamine hydrochloride, 4.0g (0.10mol) NaOH and 4.6g (0.20mol) LiOH were put into 120mL EtOH and 30mL H 2 O in a 250mL single-necked bottle. After the mixture was stirred at 0°C for 10 minutes, 11.8 g (110 mmol) of bromoethane was added dropwise to the mixture keeping the temperature below 5°C. After the addition, the system was slowly warmed up to 35°C and continued to stir for 24 hours. GC detected that the reaction was complete. After removing a large amount of inorganic salts by filtration, the filtrate was spin-dried to remove a large amount of ethanol. To the concentrate was added 40 mL of water and extracted with dichloromethane (3 x 60 mL). Organic phase with or without NaSO 4 Dry and concentrate under reduced pressure to obtain 6.5 g of yellow oil 2-(ethylthio)ethylamine with a yield of 61%.
[0065] The structural confirmation data are...
Embodiment 11
[0098] Example 11 The compound Mn-4 represented by formula 1 catalyzes the dehydrogenation coupling of ampicol (1a) and cycloheptanone (2a) into 7,8,9,10-tetrahydro-6H-cyclohepta[b]quinoline (3aa)
[0099] in N 2 Under atmosphere, put 61mg (0.5mmol) o-aminobenzyl alcohol, 112mg (2eq., 1.0mmol) cycloheptanone, 4.5mg (10umol, 2mol%) Mn-4, 1.0mmol (2eq.) base promoter into 20mL Schlenk bottle, and add a certain amount (2.0 ~ 4.0mL) toluene and (0.5 ~ 1.0mL) tetrahydrofuran. The reaction mixture was stirred at 120°C for 48-72 hours. Cool to room temperature, add 5 mL saturated NH 4 The Cl aqueous solution was detected and analyzed by GC (using mesitylene as an internal standard) and the results are shown in Table 1.
[0100]
[0101] Table 1: Results of 3aa prepared by dehydrocoupling reaction under different cocatalysts a .
[0102]
[0103]
[0104] a Reaction conditions: 0.5mmol o-aminobenzyl alcohol, 1.0mmol cycloheptanone, 10umol Mn-4, 1.0mmol base additive, 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com