Luminescent tetradentate ligand-containing gold (III) compounds for organic light-emitting devices and their preparation
A compound and light-emitting layer technology, which is applied in the direction of gold-organic compounds, organic chemistry, and compounds containing elements of Group 1/11 of the periodic table, can solve problems such as low efficiency and low operating voltage, reduce non-radioactive decay, improve Product yield, effect of improving chemical and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
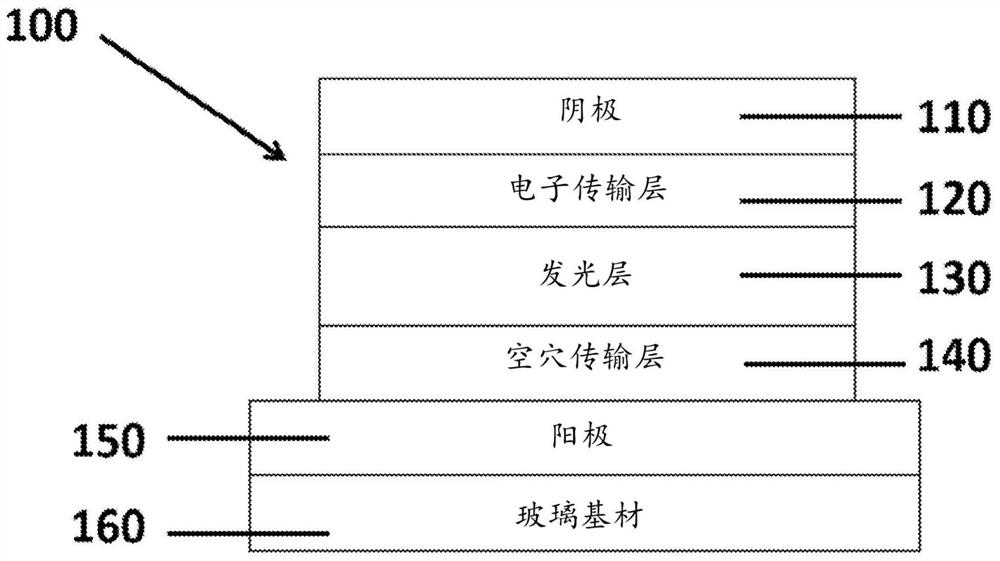
[0095] Embodiments of the subject matter described herein relate to thermally stable gold(III) compounds containing highly rigid tetradentate ligands. Other embodiments of the subject matter described herein relate to the modification of luminescent tetradentate ligand-containing gold(III) compounds. The luminescent gold(III) compound comprises a tetradentate ligand containing four attachment sites coordinated to the gold(III) metal center. The attachment site consists of a coordinating atom, which may be, for example, nitrogen, carbon, or a sigma-donor group, eg, an aryl group.
[0096] Luminescent gold(III) compounds containing tetradentate ligands include:
[0097] at least one gold metal center having a +3 oxidation state and four coordination sites;
[0098] A tetradentate ligand with a coordinating atom or functional group as the linking site for coordination to the gold center; and the coordinating site is linked directly or via an aromatic system or spacer to form a ...
Embodiment 1
[0144] As described in Scheme 1, compounds 1-7 were prepared according to the following methods. All tetradentate ligand-containing gold(III) compounds were synthesized by cyclometallation of gold(III) precursors in a one-pot process in the presence of a catalytic amount of palladium catalyst in base and organic solvent The compound or its equivalent is reacted with the corresponding heterocycle. For example, compound 1 was synthesized by combining [Au{C^(4-C 6 h 5 )C^NBr-6}Cl] (276mg, 0.45mmol) and 3,6-di-tert-butyl-1-(4,4,5,5-tetramethyl-1,3,2-dioxa A mixture of cyclopentaboran-2-yl)-9H-carbazole (138 mg, 0.45 mmol), palladium catalyst and base was stirred overnight at reflux temperature in a degassed solvent (Scheme 1). After removal of the solvent, the crude product was purified by dissolving in dichloromethane with slow diffusion of diethyl ether. The solid was then filtered and dried in vacuo to give a yellow solid (102 mg). NMR spectra were recorded on a Bruker AVA...
Embodiment 2
[0158] UV-visible light absorption properties
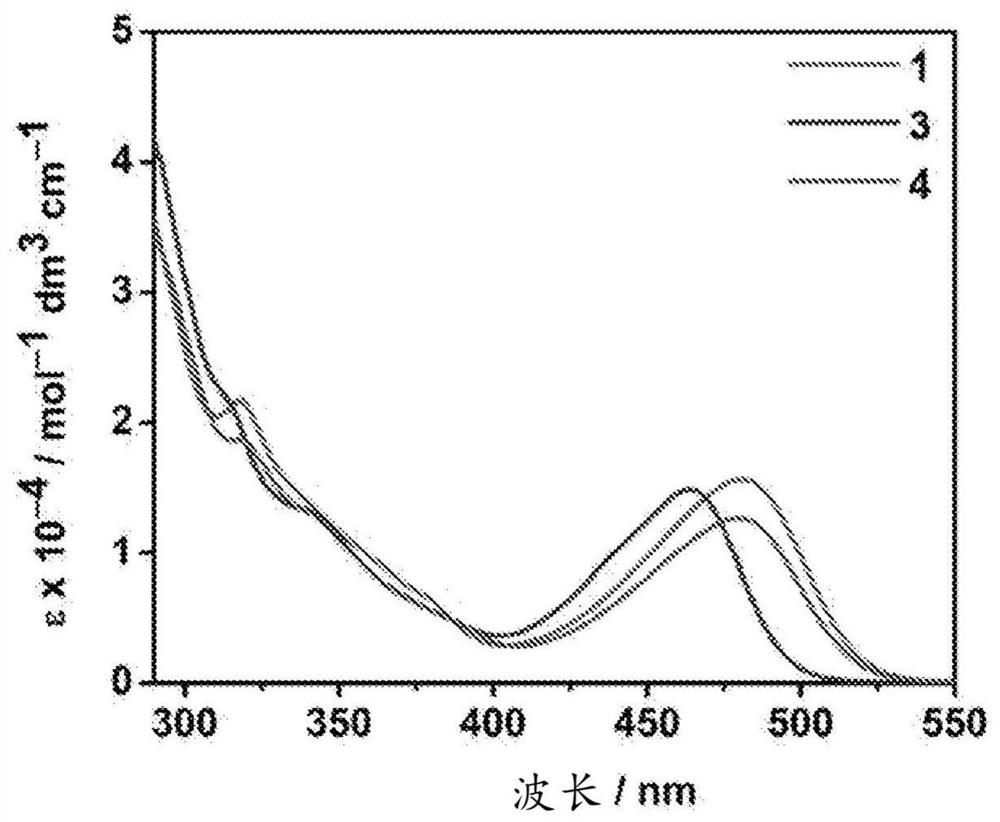
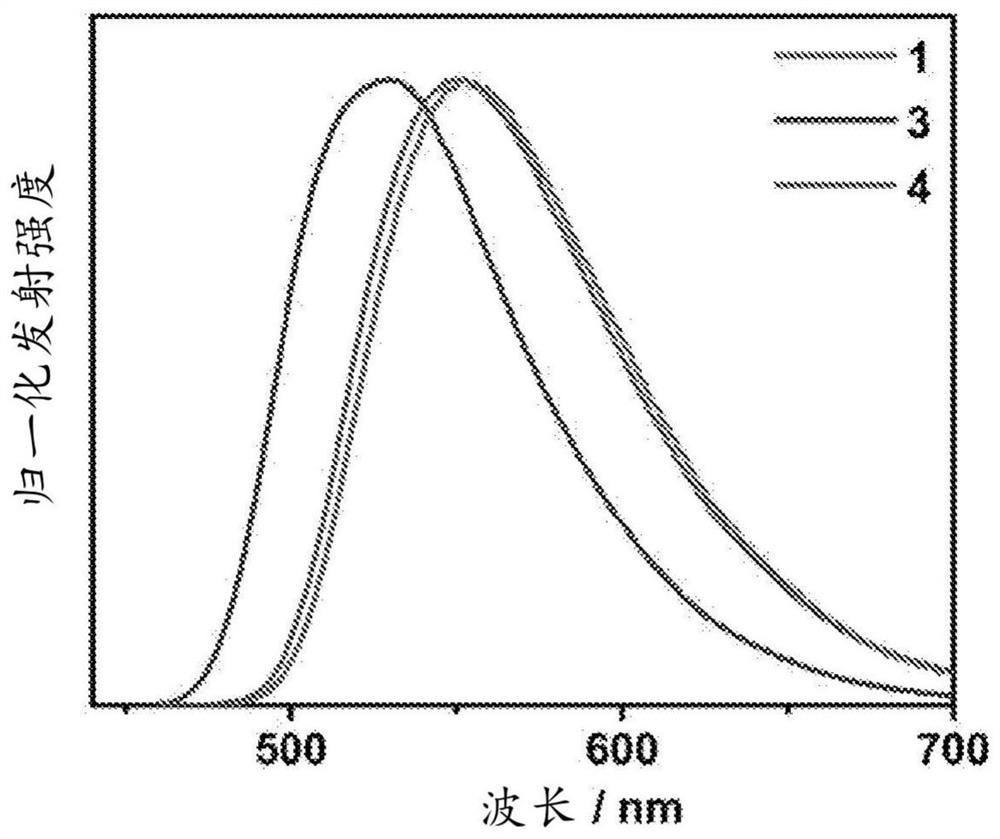
[0159] The UV-Vis absorption spectra of compounds 1, 3 and 4 in toluene solution at 298K are characterized by a moderately intense vibrational structure absorption band at about 280-350 nm, with extinction coefficients (ε) in the range of 10 4 dm 3 mol –1 cm –1 magnitude ( figure 2 ). These absorption bands are tentatively assigned to the intra-ligand (IL) [π→π*] transitions of the cyclometallated ligands and carbazole moieties. On the other hand, a broad and moderately intense unstructured absorption band was observed for all complexes at about 410–530 nm. These absorption bands are tentatively assigned to metal-perturbed IL[π→π*] transitions and IL charge transfer (ILCT)[π(carbazole)→ π*(pyridine)] transition composition. Due to the non-reducing nature of the Au(III) center, metal-to-ligand charge-transfer transitions (MLCT) to higher oxidation states of Au(IV) are unlikely to be achieved. The UV-Vis absorption data o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com